Summary
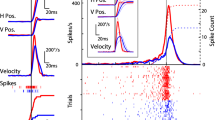
Cells of origin of the corpus callosum (callosal efferent neurons) in prelunate gyrus (area OA) of the rhesus monkey were studied using electrophysiological techniques. Monkeys were chronically prepared and callosal efferent neurons were identified by their antidromic activation following electrical stimulation of the contralateral prelunate gyrus and/or the splenium of the corpus callosum. Interhemispheric antidromic latencies ranged from 2.6–18.0 ms (median = 7.0 ms) while the conduction velocity along the length of the axon ranged from 2.8 to 22.5 M/s (median = 7.4 M/s). Following the relative refractory period of a single prior impulse, all but one of 61 callosal efferent neurons studied showed a supernormal period of increased axonal conduction velocity and excitability. Following several prior impulses, the supernormal period was followed by a subnormal period of decreased axonal conduction velocity and excitability, which, depending on the number of prior impulses, lasted from several hundred ms to nearly 2 min.
Similar content being viewed by others
References
Bergmans, J.: Physiological observations on single human nerve fibres. In: “New Developments in Electromyography and Clinical Neurophysiology” Desmedt, J.E. ed, Vol. 2, pp. 89–127. Basel: Karger 1973
Bishop, P.O., Burke, W., Davis, R.: Single-unit recording from antidromically activated optic radiation neurons. J Physiol (Lond) 162, 432–450 (1962)
Bliss, T.V.P., Rosenberg, M.E.: Supernormal conduction velocity in the olfactory nerve of the tortoise. J Physiol (Lond) 239, 60–61 (1974)
Bonin, G.V., Bailey, P.: The Neocortex of Macaca Mulatta. Urbana: University of Illinois Press 1947
Bullock, T.H.: Facilitation of conduction rate in nerve fibers. J Physiol (Lond) 114, 89–97 (1951)
Chung, S., Raymond, S.A., Lettvin, J.Y.: Multiple meaning in single visual units. Brain Behav Evol 3, 72–101 (1970)
Fuller, J.H., Schlag, J.D.: Determination of antidromic excitation by the collision test: Problems of interpretation. Brain Res 122, 283–298 (1976)
Gardner-Medwin, A.R.: An extreme supernormal period in cerebellar parallel fibers. J Physiol (Lond) 22, 357–371 (1972)
Gilliatt, R.W., Willison, R.G.: The refractory and supernormal periods of the human median nerve. J Neurol Neurosurg Psychiat 26, 136–143 (1963)
Grossman, T., Spira, M.W., Parnas, I.: Differential flow of information into branches of a single axon. Brain Res 64, 379–386 (1973)
Hirano, A., Dembitzer, H.M.: A structural analysis of the myelin sheath in the central nervous system. J Cell Biol 34, 555–567 (1967)
Hubbard, J.I., Willis, W.D.: Hyperpolarization of mammalian motor nerve terminals. J Physiol (Lond), 163, 115–137 (1962)
Hubbard, J.I., Willis, W.D.: The effects of depolarization of motor nerve terminals upon the release of transmitter by nerve impulses. J Physiol (Lond), 194, 381–405 (1968)
Kocsis, J.D., VanderMaelen, C.P., Kitai, S.T.: Conduction properties of caudate efferent neurons in the cat. Neurosci Abstr Vol. III, p. 40 (1977)
Kuypers, H.G.J.M., Szwarcbart, M.R., Mishkin, M., Rosvold, H.E.: Occipito-temporal cortico-cortical connections in the rhesus monkey. Exp Neurol 11, 245–262 (1965)
Lass, Y., Abeles, M.: Transmission of information by the axon: I. Noise and memory in the myelinated nerve fiber of the frog. Biol Cyb 19, 61–67 (1975)
Newman, E.A., Raymond, S.A.: Activity dependent shifts in excitability of frog peripheral nerve axons. Quart Prog Rept, M.I.T. Res Lab Electr 102, 165–187 (1971)
Palay, S.L., Chan-Palay, V.: The Cerebellar Cortex: Cytology and Organization. New York: Springer 1974
Phillips, C.G.: Actions of antidromic pyramidal volleys on single Betz cells in the cat. Quart J Exp Physiol 44, 1–25 (1959)
Phillips, C.G., Powell, T.P.S., Shepherd, G.M.: Responses of mitral cells to stimulation of the lateral olfactory tract in the rabbit. J Physiol (Lond) 168, 65–88 (1963)
Raymond, S.A., Lettvin, J.Y.: After effects of activity in peripheral axons as a clue to nervous coding. In: The Physiology and Pathobiology of Axons. Waxman, S.G., (ed.). New York: Raven Press 1978
Swadlow, H.A.: Systematic variations in the conduction velocity of slowly conducting axons in the rabbit corpus callosum. Exp. Neurol 43, 445–451 (1974b)
Swadlow, H.A.: Properties of antidromically activated callosal neurons and neurons responsive to callosal input in rabbit binocular cortex. Exp Neurol 43, 424–444 (1974a)
Swadlow, H.A., Waxman, S.G.: Observations on impulse conduction along central axons. Proc Nat Acad Sci (Wash) 72, 5156–5159 (1975)
Swadlow, H.A., Waxman, S.G.: Variations in conduction velocity and excitability following single and multiple impulses of visual callosal axons in the rabbit. Exp Neurol 53, 128–150 (1976)
Swadlow, H.A., Waxman, S.G., Rosene, D.L.: Latency variability and the identification of antidromically activated neurons in mammalian brain. Exp Brain Res 32, 439–443 (1978)
Takahashi, K.: Slow and fast groups of pyramidal tract cells and their respective membrane properties. J Neurophysiol 28, 908–924 (1965)
Takeuchi, A., Takeuchi, N.: Electrical changes in the pre- and postsynaptic axons of the giant synapse of Logio. J Gen Physiol 45, 1181–1193 (1962)
Van Essen, D.C.: The contribution of membrane hyperpolarization to adaptation and conduction block in sensory neurons of the leech. J Physiol (Lond) 230, 509–549 (1973)
Waxman, S.G., Swadlow, H.A.: Morphology and physiology of visual callosal axons: Evidence for a supernormal period in central myelinated axons. Brain Res 113, 179–187 (1976a)
Waxman, S.G., Swadlow, H.A.: Ultrastructure of visual callosal axons in the rabbit. Exp Neurol 53, 115–128 (1976b)
Wilson, M.: Visual Function: Extrastriate-Pulvinar system. In: Handbook in Behavioral Neurobiology. Masterton, R.B. (ed.), Vol. 1. Sensory Integration. New York: Plenum Press
Yau, K.: Receptive fields, geometry, and conduction block of sensory neurons in the central nervous system of the leech. J Physiol (Lond) 263, 513–538 (1976)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Swadlow, H.A., Rosene, D.L. & Waxman, S.G. Characteristics of interhemispheric impulse conduction between prelunate gyri of the rhesus monkey. Exp. Brain Res. 33, 455–467 (1978). https://doi.org/10.1007/BF00235567
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00235567