Abstract
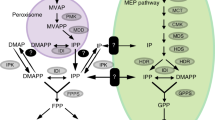
The subcellular compartmentation of isopentenyl diphosphate (IPP) synthesis was examined in secretory cells isolated from glandular trichomes of peppermint (Mentha x piperita L. cv. Black Mitcham). As a consequence of their anatomy and the conditions of their isolation, the isolated secretory cells are non-specifically permeable to low-molecular-weight water-soluble metabolites. Thus, the cytoplasm is readily accessible to the exogenous buffer whereas the selective permeability of subcellular organelles is maintained. With the appropriate choice of exogenous substrates, this feature allows the assessment of cytoplasmic and organellar (e.g. plastidic) metabolism in situ. Glycolytic substrates such as [14C]glucose-6-phosphate and [14C]pyruvic acid are incorporated into both monoterpenes and sesquiterpenes with a monoterpene:sesquiterpene ratio that closely mimics that observed in vivo, indicating that the correct subcellular partitioning of these substrates is maintained in this model system. Additionally, exogenous [14C]mevalonic acid and [14C]IPP, which are both intitially metabolized in the cytoplasm, produce an abnormally high proportion of sesquiterpenes. In contrast, incubation with either [14C]citrate or [14C]acetyl-CoA results in the accumulation of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) with no detectable isoprenoids formed. Taken together, these results indicate that the cytoplasmic mevalonic acid pathway is blocked at HMG-CoA reductase and that the IPP utilized for both monoterpene and sesquiterpene biosynthesis is synthesized exclusively in the plastids.
Similar content being viewed by others
Abbreviations
- DMAPP:
-
dimethylallyl diphosphate
- FPP:
-
farnesyl diphosphate
- GLC:
-
gas liquid chromatography
- GPP:
-
geranyl diphosphate
- HMG-CoA:
-
3-hydroxy-3-methylglutaryl-CoA
- IPP:
-
isopentenyl diphosphate
- PEP:
-
phosphoenolpyruvic acid
References
Amelunxen, F. (1965) Elektronenmikroskopische Untersuchungen an den Drüsenschuppen von Mentha piperita L. Planta Med. 13, 457–473
Amelunxen, F., Wahlig, T., Arbeiter, H. (1969) Über den Nachweis des ätherischen Öls in isolierten Drüsenhaaren und Drüsenschuppen von Mentha piperita L. Z. Pflanzenphysiol. 61, 68–72
Burden R.S., Cooke, D.T., Carter, G.A. (1989) Inhibitors of sterol biosynthesis and growth in plants and fungi. Phytochemistry 28, 1791–1804
Cheniclet, C., Carde, J.-P. (1985) Presence of leucoplasts in secretory cells and of monoterpenes in the essential oil: a correlative study. Isr. J. Bot. 34, 219–238
Coates, R.M., Denissen, J.F., Croteau, R.B., Wheeler, C.J. (1987) Geminal dimethyl stereochemistry in the enzymatic cylization of geranyl pyrophosphate to (+)- and (−)-α-pinene. J. Am. Chem. Soc. 109, 4399–4401
Connolly, J.D., Hill, R.A. (1992) Dictionary of Terpenoids, Chapman and Hall, New York
Croteau, R. (1987) Biosynthesis and catabolism of monoterpenoids. Chem. Rev. 87, 929–954
Croteau, R., Martinkus, C. (1979) Metabolism of monoterpenes. Demonstration of (+)-neomenthyl-β-d-glucoside as a major metabolite of (−)-menthone in peppermint (Mentha piperita). Plant Physiol. 64 169–175
Croteau, R.B., Satterwhite, D.M. (1990) Method for radio-capillary gas chromatography employing a modified oxidation-reduction train and flow-through detector. J. Chromatogr. 500, 349–54
Gershenzon, J., Croteau, R. (1991) Terpenoids. In: Herbivores: Their interactions with secondary plant metabolites. The Chemical Participants. Vol. I, pp. 165–219, Rosenthal, G.A., Berenbaum, M.R. eds. Academic Press, San Diego
Gershenzon, J., Croteau, R. (1993) Terpenoid biosynthesis: The basic pathway and formation of monoterpenes, sesquiterpenes and diterpenes. In: Lipid Metabolism in Plants, pp. 340–388, Moore, Jr. T.S. ed. CRC Press, Boca Raton, Fla.
Gershenzon, J., McCaskill, D., Rajaonarivony, J.I.M., Mihaliak, C., Karp, F., Croteau, R. (1992) Isolation of secretory cells from plant glandular trichomes and their use in biosynthetic studies of monoterpenes and other gland products. Anal. Biochem. 200, 130–138
Gray, J.C. (1987) Control of isoprenoid biosynthesis in higher plants. Adv. Bot. Res. 14, 25–91
Hanson, J.B. (1985) Membrane transport systems of plant mitochondria. In: Encyclopedia of plant physiology. N.S. Vol. 18: Higher plant cell respiration, pp. 248–280, Douce, R., Day, D.A. eds. Springer-Verlag, New York
Heintze, A., Görlach, J., Leuschner, C., Hoppe, P., Hagelstein, P., Schulze-Siebert, D, Schultz, G. (1990) Plastidic isoprenoid synthesis during chloroplast development. Plant Physiol. 93, 1121–1127
Huang, L.-S., Romani, R.J. (1991) Metabolically driven self-restoration of energy-linked functions by avocado mitochondria. Plant Physiol. 95, 1096–1105
Kaethner, T.M., ap Rees, T. (1985) Intracellular location of ATP citrate lyase in leaves of Pisum sativum L. Planta 163, 290–294
Kleinig, H. (1989) The role of plastids in isoprenoid biosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40, 39–59
Lawrence, B.M., Hogg, J.W., Terhune, S.J. (1972) Essential oils and their trace constituents. X. Some new trace constituents in the oil of Mentha piperita L. Flav. Indust. 3, 467–472
Liedvogel, B. (1986) Acetyl coenzyme A and isopentenylpyrophosphate as lipid precursors in plant cells — biosynthesis and compartmentation. J. Plant Physiol. 124, 211–222
McCaskill, D., Croteau, R. (1993) Procedures for the isolation and quantification of the intermediates of the mevalonic acid pathway. Anal. Biochem. 215, 142–149
McCaskill, D., Gershenzon, J., Croteau, R. (1992) Morphology and monoterpene biosynthetic capabilities of secretory cell clusters isolated from glandular trichomes of peppermint (Mentha piperita L.). Planta 187, 445–454
Munck, S.L., Croteau, R. (1990) Purification and characterization of the sesquiterpene cyclase patchoulol synthase from Pogostemon cablin. Arch. Biochem. Biophys. 282, 58–64
Ohlrogge, J.B., Jaworski, J.G., Post-Beittenmiller, D. (1993) De novo fatty acid biosynthesis. In: Lipid metabolism in plants, pp. 3–32, Moore, Jr., T.S., ed. CRC Press, Boca Raton
Peterson, J.A., Prough, R.A. (1986) Cytochrome P-450 reductase and cytochrome b5 in cytochrome P-450 catalysis. In: Cytochrome P-450. Structure, mechanism and biochemistry, pp. 89–117, Ortiz de Montellano P.R., ed. Plenum Press, New York
Randall, D.D., Miernyk, J.A., Fang, T.K., Budde, R.J.A., Schuller, K.A. (1989) Regulation of the pyruvate dehydrogenase complexes in plants. In: Annals of the New York Academy of Sciences, pp. 192–205, Roche, T.E., Patel, M.S., eds. New York
Rohmer, M., Knani, M., Simonin, P., Sutter, B., Sahm, H. (1993) Isoprenoid biosynthesis in bacteria: a novel pathway for the early steps leading to isopentenyl diphosphate. Biochem. J. 295, 517–524
Schneider, M.M., Hampp, R., Ziegler, H. (1977) Envelope permeability to possible precursors of carotenoid biosynthesis during chloroplast-chromoplast transformation. Plant Physiol. 60, 518–520
Srivastava, D.K., Bernhard, S.A. (1987) Biophysical chemistry of metabolic reaction sequences in concentrated enzyme solution and in the cell. Annu. Rev. Biophys. Biophys. Chem. 16, 175–204
Stermer, B.A., Bianchini, G.M., Korth, K.L. (1994) Regulation of HMG-CoA reductase acitivity in plants. J. Lipid Res. 35, 1133–1140
Wagner, K.G., Backer, A.I. (1992) Dynamics of nucleotides in plants studied on a cellular basis. In: International reviews of cytology, pp. 1–84, Jeon, K.W., Friedlander, M., eds. Academic Press, New York
Wellburn, A.R., Hampp, R. (1976) Uptake of mevalonate and acetate during plastid developmentf. Biochem. J. 158, 231–233
Werker, E., Ravid, U., Putievsky, E. (1985) Structure of glandular hairs and identification of the main components of their secreted material in some species of the Labiatae. Isr. J. Bot. 34, 31–45
Author information
Authors and Affiliations
Corresponding author
Additional information
This investigation was supported in part by U.S. Department of Energy Grant DE-FG06-88ER 13869, the Mint Industry Research Council, and Project 0268 from the Agricultural Research Center, Washington State University. We thank Jonathan Gershenzon for helpful criticisms of the manuscript and Greg Wichelns for growing the plants.
Rights and permissions
About this article
Cite this article
McCaskill, D., Croteau, R. Monoterpene and sesquiterpene biosynthesis in glandular trichomes of peppermint (Mentha x piperita) rely exclusively on plastid-derived isopentenyl diphosphate. Planta 197, 49–56 (1995). https://doi.org/10.1007/BF00239938
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00239938