Summary
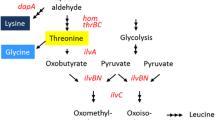
A strain of Corynebacterium glutamicum was isolated that accumulated up to 44 g/l of L-lysine-HCl from 100 g/l of glucose × H2O in a simple mineral salts medium. This strain was obtained from the wildtype by two mutagenesis steps. In the first step the aminoethyl-cysteine-resistant strain MH20 was obtained and in the second step the Leu — derivative MH20-22B. Enzymatic analysis of the hyperproducer MH20-22B revealed that this strain has feedback-resistant aspartate kinase and is devoid of isopropylmalate dehydratase. In addition, this strain has an extraordinarily high secretion rate of lysine (0.57 mmol/g dry weight and h), whereas strain MH20 has a low secretion rate (0.19 mmol/g per hour), and both strains have comparable cytosolic lysine concentrations. This suggests that the secretory step is influenced n the hyperproducer. Applying gene-directed mutagenesis, the aspartate kinase gene of the isolated strain (coding for feedback-resistant enzyme) was replaced by the gene coding of feedback-sensitive wild-type enzyme. The resulting strain still secretes lysine, although in low amounts (≤2 g/l). This is proof of the superior role of kinase regulation in metabolite flow and is indicative of unknown mutations, one of which is probably in the secretory system.
Similar content being viewed by others
References
Abe S, Takayama K, Kinoshita S (1967) Taxonomical studies on glutamic acid-producing bacteria. J Gen Appl Microbiol Tokyo 13:279–301
Adelberg EA, Mandel M, Chen GCC (1965) Optimal conditions for mutagenesis by N-methyl-N′-nitro-N-nitrosoguanidine in Escherichia coli K12. Biochem Biophys Res Comm 18:788–795
Birnboim HC, Doly J (1979) A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res 7:1513–1523
Bröer S, Krämer R (1991a) Lysine excretion by Corynebacterium glutamicum 1. Identification of a specific secretion carrier system. Eur J Biochem 202:131–135
Bröer S, Krämer R (1991b) Lysine excretion by Corynebacterium glutamicum 2. Energetics and mechanism of the transport system. Eur J Biochem 202:137–143
Cremer J, Treptow C, Eggeling L, Sahm H (1988) Regulation of enzymes of lysine biosynthesis in Corynebacterium glutamicum. J Gen Microbiol 134:3221–3229
Cremer J, Eggeling L, Sahm H (1991) Control of the lysine biosynthetic sequence in Corynebacterium glutamicum as analyzed by overexpression of the individual corresponding genes. Appl Environ Microbiol 57:1746–1752
Eikmanns BJ, Follettie MT, Griot MU, Sinskey AJ (1989) The phosphoenolpyruvate carboxylase gene of Corynebacterium glutamicum: molecular cloning, nucleotide sequence, and expression. Mol Gen Genet 218:330–339
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580
Kalinowski J, Cremer J, Bachmann B, Eggeling L, Sahm H, Pühler A (1991) Genetic and biochemical analysis of the aspartolinase from Corynebacterium glutamicum. Mol Microbiol 5:1197–1204
Kleemann A, Leuchtenberger W, Hoppe B, Tanner H (1985) Amino acids. In: Gerhartz W (ed) Ullmannss encyclopedia of industrial chemistry, vol 2A. VCH Verlagsgesellschaft, Weinheim, pp 57–97
Kohlhaw G (1988) Isopropylmalate dehydratase from yeast. Methods Enzymol 166:423–429
Kohlhaw G (1989) α-Isopropylmalate synthase from yeast. Methods Enzymol 166:414–423
Menkel E, Thierbach G, Eggeling L, Sahm H (1989) Influenceof increased aspartate availability on lysine formation by a recombinant strain of Corynebacterium glutamicum and utilization of fumarate. Appl Environ Microbiol 55:684–688
Mori M, Shiio I (1986) Multiple interaction of fructose 1,6 bisphosphate and other effectors on phosphoenolpyruvate carboxylase from Brevibacterium flavum and its aspartate-producing mutants. Agric Biol Chem Tokyo 50:2605–2614
Nakayama K (1985) Lysine. In:Moo-Young M (ed) Comprehensive biotechnology, vol 3. Pergamon Press, Oxford, pp 607–620
Ozaki H, Shiio I (1983) Production of lysine by pyruvate kinase mutants of Brevibacterium flavum. Agric Biol Chem Tokyo 47:1569–1576
Parsons SJ, Burns RO (1970) β-Isopropylmalate dehydrogenase. Methods Enzymol 17A:793–799
Riddles PW, Blakeley RL, Zerner B (1979) Ellman's reagent: 5,5′ dithiobis(2-nitrobenzoic acid) — a reexamination. Anal Biochem 94:75–81
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning, a laboratory manual second edition. Cold Spring Harbour Laboratory Press
Sano K, Shioo I (1970) Microbial production of L-lysine III. Production by mutants resistant S-(2-aminoethyl)-L-cysteine. J Gen Appl Microbiol 16:373–391
Schäfer A, Kalinowski J, Simon R, Seep-Feldhaus A, Pühler A (1990) High-frequency conjugal plasmid transfer from Gram-negative Escherichia coli to various Gram-positive coryneform bacteria. J Bacteriol 172:1663–1666
Schrumpf B, Schwarzer A, Kalinowski J, Pühler A, Eggeling L, Sahm H (1991) A functionally split pathway for lysisne synthesis in Corynebacterium glutamicum. J Bacteriol 173:4510–4516
Schwarzer A, Pühler A (1990) Genetic manipulation of the amino acid-producing Corynebacterium glutamicum strain ATCC 13032 by gene disruption and gene replacement. Bio/Technology: 9:84–87
Shiio I, Ozaki H, Ujigawa-Takeda K (1982) Production of aspartic acid and lysine by citrate synthase mutants of Brevibacterium flavum. Agric Biol Chem Tokyo 46:101–107
Shiio I, Yoshino H, Sugimoto S (1990) Isolation and properties of lysine-producing mutants with feedback-resistant aspartokinase derived from a Brevibacterium flavum strain with citrate synthase- and pyruvate kinase-defects and feedback-resistant phosphoenolpyruvate carboxylase. Agric Biol Chem Tokyo 54:3275–3282
Simon R, Priefer U, Pühler A (1983) A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technol 1:784–791
Stephanopoulos G, Vallino JJ (1991) Network rigidity and metabolic engineering in metabolite overproduction. Science 252:1675–1681
Tosaka O, Takinami K, Hirose Y (1978a) L-Lysine production by S-(2-aminoethyl)l-cysteine and α-amino-β-hydroxyvaleric acid resistant mutants of Brevibacterium lactofermentum. Agric Biol Chem Tokyo 42:745–752
Tosaka O, Hirakawa H, Takinami K, Hirose Y (1978b) Regulation of lysine biosynthesis by leucine in Brevibacterium lactofermentum. Agric Biol Chem Tokyo 42:1501–1506
Wetzma PD (1969) Citrate synthase from Escherichia coli. Methods Enzymol 13:22–26
Author information
Authors and Affiliations
Additional information
Correspondence to: L. Eggeling
Rights and permissions
About this article
Cite this article
Schrumpf, B., Eggeling, L. & Sahm, H. Isolation and prominent characteristics of an L-lysine hyperproducing strain of Corynebacterium glutamicum . Appl Microbiol Biotechnol 37, 566–571 (1992). https://doi.org/10.1007/BF00240726
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00240726