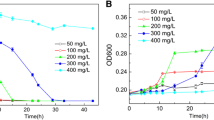
Abstract
Both Alcaligenes eutrophus JMP 134 and its plasmid-free derivative Alcaligenes eutrophus JMP 222 utilize 2,6-dinitrophenol as sole source of carbon, energy, and nitrogen. In the presence of ammonia resting cells of these strains release two mol of nitrite per mol of 2,6-dinitrophenol. Alcaligenes eutrophus JMP 222-α1D, a mutant of strain JMP 222 obtained by transposon (Tn5) mutagenesis, is able to use 2,6-dinitrophenol as nitrogen source but not as source of carbon and energy. Resting cells of this mutant liberate only one mol of nitrite per mol of 2,6-dinitrophenol. A single metabolite was detected by high-pressure liquid chromatography and identified as 2-hydroxy-5-nitropenta-2,4-dienoic acid from the mass spectrum, the 1H-, and 13C-NMR spectra. Strain JMP 222-α1S, a spontaneous mutant of strain JMP 222-α1D, accumulates 4-nitropyrogallol which was identified as the initial metabolite of 2,6-dinitrophenol degradation.
Similar content being viewed by others
Abbreviations
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- 2,6-DNP:
-
2,6-dinitrophenol
- HNMA:
-
2-hydroxy-5-nitromuconic acid
- HNPA:
-
2-hydroxy-5-nitropenta-2,4-dienoic acid
- NB:
-
nutrient broth
- NMR:
-
nuclear magnetic resonance
- NPG:
-
4-nitropyrogallol
- O.D.:
-
optical density
- tR :
-
retention time
- UV/Vis:
-
ultraviolet/visible
References
Bruhn C, Lenke H, Knackmuss H-J (1987) Nitrosubstituted aromatic compounds as nitrogen source for bacteria. Appl Environ Microbiol 53: 208–210
Dorn E, Hellwig M, Reineke W, Knackmuss H-J (1974) Isolation and characterization of a 3-chlorobenzoate degrading pseudomonad. Arch Microbiol 99: 61–70
Einhorn A, Cobliner J, Pfeiffer H (1904) Über das Pyrogallol. Chem Ber 37: 113–114
Germanier F, Wuhrmann K (1963) Über den aeroben mikrobiellen Abbau aromatischer Nitroverbindungen. Pathol Microbiol 26: 569–578
Grosjean D (1985) Reactions of o-cresol and nitrocresol with NOx in sunlight and with ozone-nitrogen dioxide mixtures in the dark. Environ Sci Technol 19: 968–974
Gundersen K, Jensen HL (1956) A soil bacterium decomposing organic nitro-compounds. Acta Agric Scand 6: 100–114
Hallas LE, Alexander M (1983) Microbial transformation of nitroaromatic compounds in sewage effluent. Appl Environ Microbiol 45: 1234–1241
Hess TF, Schmidt SK, Silverstein J, Howe B (1990) Supplemental substrate enhancement of 2,4-dinitrophenol mineralization by a bacterial consortium. Appl Environ Microbiol 56: 1551–1558
Jensen HL, Lautrup-Larsen G (1967) Microorganisms that decompose nitroaromatic compounds with special reference to dinitroortho-cresol. Acta Agricol Scand 17: 115–126
Kearney PC, Kaufman DD (1975) Herbicides chemistry, degradation and mode of action. Marcel Dekker, New York
Montgomery HAC, Dymock JF (1961) The determination of nitrite in water. Analyst 86: 414–416
Murray NE, Brammar WJ, Murray K (1977) Lamboid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet 150: 53–61
Pemberton JM, Corney B, Don RH (1979) Evolution and spread of pesticide degrading ability among soil micro-organisms. In: Timmis KN, Pühler A (eds) Plasmids of medical, environmental and commerical importance. Elsevier/North Holland Biomedical Press, Amsterdam, pp 287–299
Pfennig N, Lippert KD (1966) Über das Vitamin B12-Bedürfnis phototropher Schwefelbakterien. Arch Mikrobiol 55: 245–256
Schmidt SK, Gier MJ (1989) Dynamics of microbial populations in soil: Indigenous microorganisms degrading 2,4-dinitrophenol. Microbiol Ecol 18: 285–296
Simpson JR, Evans WC (1953) The metabolism of nitrophenols by certain bacteria. Biochem J 55: XXIV
Spain JC, Wyss O, Gibson DT (1979) Enzymatic oxidation of p-nitrophenol. Biochem Biophys Res Commun 88: 634–641
Tewfik MS, Evans WC (1966) DNOC-metabolism. Biochem J 99: 31P
Zeyer J, Kearney PC (1984) Degradation of o-nitrophenol and m-nitrophenol by a Pseudomonas putida. J Agric Food Chem 32: 238–242
Zeyer J, Kocher HP, Timmis KN (1986) Influence of parasubstituents on the oxidative metabolism of o-nitrophenols by Pseudomonas putida B2. Appl Environ Microbiol 52: 334–339
Zeyer J, Kocher HP (1988) Purification and characterization of a bacterial nitrophenol oxygenase which converts ortho-nitrophenol to catechol and nitrite. J Bacteriol 170: 1789–1794
Zoeteman BCJ, Harmsen K, Linders JBHJ, Morra CFH, Slooff W (1980) Persistent organic pollutants in river water and ground water of the Netherlands. Chemosphere 9: 231–249
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ecker, S., Widmann, T., Lenke, H. et al. Catabolism of 2,6-dinitrophenol by Alcaligenes eutrophus JMP 134 and JMP 222. Arch. Microbiol. 158, 149–154 (1992). https://doi.org/10.1007/BF00245219
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00245219