Abstract
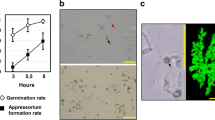
Appressorium formation in germinating Colletotrichum gloeosporioides is induced by the surface wax of the host, the avocado fruit. To elucidate the mechanism by which differentiation of appressorium formation is induced, the fungal genes specifically activated by this host signal were sought. From a cDNA library of the transcripts present in appressorium-forming conidia, the clones representing nongerminating conidia were removed by hybridization with cDNAs synthesized from the nongerminating conidia. From this subtracted library, clones that hybridized with cDNA for transcripts from appressorium-forming conidia and not with cDNA for transcripts from germinating conidia were selected. Three such clones were isolated and sequenced. The genes for these three transcripts were also cloned and sequenced. Northern blot analysis showed that transcripts that hybridized with these three clones were expressed in the conidium only during the process of appressorium formation induced by avocado surface wax, and that these transcripts were not detectable when appressorium formation was prevented even in the presence of avocado wax. Nucleotide sequences of the clones revealed that one clone, cap3, contained an open reading frame (ORF) that would code for a 26-amino acid, cysteine-rich peptide with significant homology to Neurospora crassa copper metallothionein. Another clone, cap5, contained an ORF that would code for a 27-amino acid cysteine-rich peptide with less homology to metallothioneins. Cu2+ and Cd2+ also induced the expression of these genes at lower levels. The histochemical analysis of transformants containing the cap5 promoter fused to the β-glucuronidase (GUS) gene showed that the cap5 gene promoter caused GUS expression exclusively during appressorium formation and most of the gus activity was in the appressorium. The cap22 clone contained an ORF coding for a 227-amino acid polypeptide of 22 kDa, which did not show significant homology to any known proteins. Recombinant CAP22 protein was produced using a pET-19b expression system in Escherichia coli, purified, and used to prepare rabbit antibodies. Western blot analysis of proteins from the appressorium-forming conidia revealed a major cross-reacting protein at 43 kDa and a minor band at 68 kDa, indicating that the potential glycosylation sites found in the primary translation product were probably glycosylated. Results of immunogold localization showed that CAP22 protein was located on the wall of the appressorium.
Similar content being viewed by others
References
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1992a) Current protocols in molecular biology, vol 1. John Wiley Interscience, New York, pp 4.2.3–4.2.4
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Strühl K (1992b) Current protocols in molecular biology, vol. 1. John Wiley Interscience, New York, pp 5.8.6–5.8.13
Bajar A, Podila GK, Kolattukudy, PE (1991) Identification of a fungal cutinase promoter that is inducible by a plant signal via a phosphorylated trans-acting factor. Proc Natl Acad Sci USA 88:8208–8212
Beclar J, Craig EA (1994) Heat shock proteins as molecular chaperones. Eur J Biochem 219:11–23
Bhairi SM, Staples RC, Frene P, Yoder OC (1989) Characterization of an infection structure-specific gene from the rust fungus Uromyces appendiculatus. Gene 81:237–243
Bremner I (1987) Nutritional and physiological significance of metallothionein. Exp Suppl 52:81–107
Carley HE, Watson RD, Huber DM (1967) Inhibition of pigmentation in Aspergillosis niger by dimethylsulfoxide. Can J Bot 45:1451–1453
Dickinson S (1977) Studies in the physiology of obligate parasitism X. Induction of response to a thigmotropic stimulus. Phytopathology 89:97–115
Dickinson S (1979) Growth of Erysiphe germinis on artificial membranes. Physiol Plant Pathol 15:219–221
Edwards MC, Bowlings DJF (1986) The growth of rust germ tubes towards stomata in relation to pH gradients. Physiol Mol Plant Pathol 29:181–196
Emmett RW, Parbery DG (1975) Appressoria. Annu Rev Phytopathol 13:147–167
Hankin L, Kolattukudy PE (1968) Metabolism of a plant wax paraffin (n-Nanocosane) by a soil bacterium (Micrococcus cerificans). J Gen Microbiol 51:457–463
Harti FU, Hlodan R, Langer T (1994) Molecular chaperones in protein folding: the act of avoiding sticky situations. Trends Biochem Sci 19:20–25
Heath MC (1977) A comparative study of non-host interactions with rust fungi. Physiol Plant Pathol 10:73–88
Heath MC, Heath OB (1978) Structural studies of the development of infection structures of cowpea rust Uromyces phaseoli var. vignae. I. Nucleoli and nuclei. Can J Bot 56:648–661
Hoch HC, Staples RC (1984) Evidence that cyclic AMP initiates nuclear division and infection structure formation in the bean rust fungus, Uromyces phaseoli. Exp Mycol 8:37–46
Hoch HC, Staples RC (1991) The fungal spore and disease initiation in plants and animals. Plenum Press, New York, pp 25–46
Hoch HC, Staples RC, Bourett TM (1987a) Chemically induced appressoria in Uromyces appendiculatus are formed aerially, apart from the substrate. Mycologia 79:418–424
Hoch HC, Staples RC, Whitehead B, Comeau J, Wolfe ED (1987b) Signaling for growth orientation and cell differentiation by surface topography in Uromyces. Science 234:1659–1662
Johnson T (1934) A tropic response in germ tubes of urediospores of Puccinia graminis tritici. Phytopathology 24:80–82
Kägi JH, Schäffer A (1988) Biochemistry of metal lothionein. Biochemistry 27:8509–8515
Kubo Y, Nakamura H, Kobayashi K, Okuno T, Furusawa I (1991) A cloning of a melanin biosynthetic gene essential for appressorial penetration of Colletotrichum lagenarium. Mol PlantMicrobe Interact 4:440–445
Lapp MS, Skoropad WP (1978) Location of appressoria of Colletotrichum graminicola on natural and artificial barley leaf surfaces. Trans Br Mycol Soc 70:225–228
Lee YH, Dean RA (1993) Stage-specific gene expression during appressorium formation of Magnaporthe grisea. Exp Mycol 17:215–222
Malmstrom BG, Ryden L (1968) The copper-containing oxidases. In: Singer TP (ed) Biological oxidations. Intersciences, New York, pp 415–438
Maniatis T, Fritsch ET, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Mohan R, Bajar AM, Kolattukudy PE (1993) Induction of a tomato anionic peroxidase gene (tap1) by wounding in transgenic tobacco and activation of tap1/GUS and tap2/GUS chimeric gene fusions in transgenic tobacco by wounding and pathogen attack. Plant Mol Biol 21:341–354
Munger K, Germann VA, Lerch K (1985) Isolation and structural organization of the Neurospora crassa copper metallothionein gene. EMBO J 4:2665–2668
Parbery DG (1963) Studies on graminicolous species of phyllachora FcKl. I. Ascospores — their liberation and germination. Aust J Bot 11:117–130
Podila GK, Rogers LM, Kolattukudy PE (1993) Chemical signals from avocado surface wax trigger germination and appressorium formation in C. gloeosporioides. Plant Physiol 103:267–272
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Skoropad WP (1967) Effect of temperature on the ability of Colletotrichum graminicola to form appressoria and penetrate barley leaves. Can J Plant Sci 47:431–434
Staples RC, Macko V (1980) Formation of infection structures as a recognition response in fungi. Exp Mycol 4:2–16
Staples RC, Hoch HC (1987) Infection structure — form and function. Exp Mycol 11:163–169
Staples RC, App AA, Ricci P (1975) DNA synthesis and nuclear division during formation of infection structures by bean rust uredospores germlings. Arch Microbiol 104:123–127
Staples RC, Laccetti L, Yaniv Z (1976) Appressorium formation and nuclear division in Colletotrichum truncatum. Arch Microbiol 109:75–84
Staples RC, Hoch HC, Epstein L (1985) The development of infection structure by the rust and other fungi. Microbiol Sci 2:193–198
Sutton BC (1962) Colletotrichum oleratum (Pers. ex. Fr.) Grove and C. trichellum (Fr. ex. Fr.) Duke. Trans Br Mycol Soc 45:222–232
Thiele DJ (1988) ACE 1 regulates expression of the Saccharomyces cerevisiae metallothionein gene. Mol Cell Biol 8:2745–2752
Thiele DJ, Walling MJ, Hamer DH (1986) Mammalian metallothionein is functional in yeast. Science 231:854–856
Van Burgh P (1950) Some factors affecting appressorium formation and penetrability of Colletotrichum phomoides. Phytopathology 40:29–35
Xuei X, Bhairi S, Staples RC, Yoder OC (1992a) Characterization of INF56, a gene expressed during infection structure development of Uromyces appendiculatus. Gene 110:49–55
Xuei X, Bhairi S, Staples RC, Yoder OC (1992b) Differentiation-specific genes of rust fungi have limited distribution amoung fungi. Exp Mycol 16:320–323
Author information
Authors and Affiliations
Additional information
Communicated by C. van den Hondel
Rights and permissions
About this article
Cite this article
Hwang, CS., Kolattukudy, P.E. Isolation and characterization of genes expressed uniquely during appressorium formation by Colletotrichum gloeosporioides conidia induced by the host surface wax. Molec. Gen. Genet. 247, 282–294 (1995). https://doi.org/10.1007/BF00293196
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00293196