Abstract
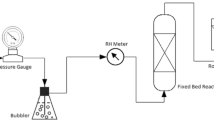
Atmospheric Hg is present in different physical and chemical forms, which determine its atmospheric transformation and transport capacities. The chemistry of Hg in flue gases is thus of importance for the deposition pattern around point source emissions. In order to apply Hg cleaning methods in flue gases its speciation is also of importance. To investigate this under realistic conditions, a 17 kW propane fired flue gas generator was used, while the kinetics of specific Hg reactions were investigated in a continuous flow reactor. Elemental Hg is readily oxidized by Cl2 and HCl both at room and at elevated temperatures (up to 900 °C) but not by NH3, N2O, SO2 or H2S. It reacts with O2 if a catalyst, such as activated carbon, is present. A slow reaction between Hg and NO2 has also been noted.
Similar content being viewed by others
References
Hall, B., Lindqvist, O. and Ljungström, E.: 1990, Environ. Sci. & Technol. 24, 108.
Lindqvist, O. and Schager P.: 1990, VDI Berichte 838, 401.
Bergström, J. G. T.: 1986, Waste Management & Research 4, 57.
Vogg, H., Braun, H., Metzger, M. and Schneider, J.: 1987, Chemospere 16, 21.
Lodenius, M. and Laaksovirte, K.: 1979, Ann. Bot. Finnici 16, 7.
Lindqvist, O. and Rodhe, H.: 1985, Tellus 37B, 136.
Lindqvist, O. : 1988, “Mercury Emissions from Swedish Waste Incineration Plants” Report OOK 88:09, ISSN 0283-8575 1–15 (In Swedish).
Mitra, S. : 1986, “Hg in the Ecosystem.”, ISBN 0-87849-529-0, Trans. Tech. Publications Ltd. Switzerland 1–327.
P'yankov, V.A.: 1949, Journal of General Chemistry of USSR. 19, 187.
Menke, R. and Wallis, G.: 1980, Am. Ind. Hyg. Assoc. J. 41, 120.
Medhekar, A.K., Rokni, M., Trainor, D.W. and Jacob, J.H.: 1979, Chemical Physics Letters 65, 600.
Cooper, D.: 1989, “Some Aspects of NOX Control in Fluidized Bed Combustion”, Thesis, Department of Inorganic Chemistry GU/CTH, Göteborg, Sweden.
Rosser, W.A. and Wise H.: 1965, J. Chem. Phys. 24, 493.
Freeman, E.S. and Gordon S.: 1956, J. Amer. Chem. Soc. 78, 1813.
Weast, R.C. : 1977–78, “CRC Handbook of Chemistry and Physics” 58th Edition.
Taylor, G.B. and Hulett, G.A.: 1913, J. Phys. Chem. 17, 565.
Braune, H. and Knoke, S.: 1931, Z. Physik. Chem. 152, 409.
Oza, T.M. and Ezekiel, E.I.: 1962, Sci. & Indus. Res. 21B, 536.
Oza, T.M., Jha, J.C. and Ezekiel, E.I.: 1968, J. Indian Chem. Soc. 1, 420.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hall, B., Schager, P. & Lindqvist, O. Chemical reactions of mercury in combustion flue gases. Water, Air, and Soil Pollution 56, 3–14 (1991). https://doi.org/10.1007/BF00342256
Issue Date:
DOI: https://doi.org/10.1007/BF00342256