Abstract
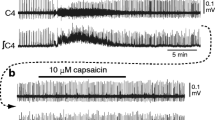
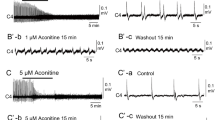
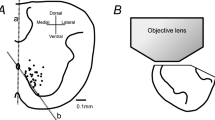
Involvement of chloride-dependent, gamma-aminobutyric acid-(GABA-) like synaptic inhibition in the generation of respiratory rhythm was studied in brainstemspinal cord preparations isolated from newborn rats. Primary respiratory rhythm is presumably generated within the rostral ventrolateral medulla, the site of Pre-I neurones, the firing of which precedes inspiration. Therefore, we examined the responses of Pre-I and inspiratory neurones to GABA antagonists (picrotoxin and bicuculline), a glycine antagonist (strychnine) and reduced chloride concentration in the perfusate. These antagonists (2–20 μM) and reduction of chloride concentration reversibly blocked the transient inhibition of Pre-I activity that occurred during the inspiratory phase. The rhythmic Pre-I and inspiratory neurone activity remained. Changes in the firing properties of Pre-I and inspiratory neurones in 10 μM bicuculline, 10 μM picrotoxin, 5 μM strychnine or reduction of chloride concentration to 40% of normal were analysed statistically. Burst rate of Pre-I neurones tended to increase during these treatments. Delay time from initiation of Pre-I firing to the peak of C4 motorneurone inspiratory activity tended to decrease except during reduced chloride concentration. Changes in mean intraburst firing frequency of Pre-I neurones were not consistent; increase (32%), no change (38%) or decrease (30%). Burst duration of inspiratory neurones decreased. Intraburst firing frequency of inspiratory neurones tended to increase except in 5 μM strychnine. GABA (0.1 mM) or glycine (0.2 mM) reduced the intraburst firing frequency and burst rate of Pre-I neurones, but did not affect the intraburst firing frequency of inspiratory neurones. The burst duration of inspiratory neurones increased during GABA and glycine treatment. The results suggest: 1. Inhibition of Pre-I activity during the inspiratory phase depends on chloride-dependent, GABA- (or glycine-) like inhibitory synaptic interaction (probably inhibitory synaptic inputs to Pre-I neurones), but Pre-I rhythm generation does not require this phasic inhibition. 2. Tonic GABAA-like inhibition (where A denotes the receptor sub-type) might be involved in the modulation of rhythm generation and inspiratory pattern generation.
Similar content being viewed by others
References
Arata A, Onimaru H, Homma I (1990) Respiration-related neurons in the ventral medulla of newborn rats in vitro. Brain Res Bull 24: 599–604
Ballantyne D, Richter DW (1984) Post-synaptic inhibition of bulbar inspiratory neurones in the cat. J Physiol (Lond) 348: 67–87
Ballantyne D, Richter DW (1986) The non-uniform character of expiratory synaptic activity in expiratory bulbospinal neurones of the cat. J Physiol (Lond) 370: 433–456
Bianchi AL, Grélot L, Iscoe S, Remmers JE (1988) Electrophysiological properties of rostral medullary respiratory neurones in the cat: an intracellular study. J Physiol (Lond) 407: 293–310
Champagnat J, Denavit-Saubie M, Moyanova S, Rondouin G (1982) Involvement of amino acids in periodic inhibitions of bulbar respiratory neurons. Brain Res 237: 351–365
Ezure K, Manabe M (1988) Decrementing expiratory neurons of the Bötzinger complex. II. Direct inhibitory synaptic linkage with ventral respiratory group neurons. Exp Brain Res 72: 159–166
Feldman JL, Smith JC (1986) Separate generation of oscillation and motor burst pattern for mammalian respiration: in vitro studies. Soc Neurosci Abstr 12: 791
Feldman JL, Smith JC (1989) Cellular mechanisms underlying modulation of breathing pattern in mammals. In: Davis M, Jacobs BL, Schoenfeld RI (eds) Modulation of defined vertebrate neural circuits. Ann NY Acad Sci 563: 114–130
Grélot L, Iscoe S, Bianchi AL (1988) Effects of amino acids on the excitability of respiratory bulbospinal neurons in solitary and para-ambigual regions of medulla in cat. Brain Res 443: 27–36
Haji A, Connelly C, Schultz SA, Wallace J, Remmers JE (1987) Postsynaptic actions of inhibitory neutrotransmitters on bulbar respiratory neurons. In: Euler C von, Langercrantz H (eds) Neurobiology of the control of breathing. Raven Press, New York, pp 187–194
Harada Y, Kuno M, Wang YZ (1985) Differential effects of carbon dioxide and pH on central chemoreceptors in the rat in vitro. J Physiol (Lond) 368: 679–693
Heyer EJ, Nowak LM, MacDonald RL (1981) Bicuculline: a convulsant with synaptic and nonsynaptic actions. Neurology 31: 1381–1390
Lindsey BG, Segers LS, Shannon R (1987) Functional associations among simultaneously monitored lateral medullary respiratory neurons in the cat. II. Evidence for inhibitory actions of expiratory neurons. J Neurophysiol 57: 1101–1117
Merrill EG, Lipski J, Kubin L, Fedorko L (1983) Origin of the expiratory inhibition of nucleus tractus solitarius inspiratory neurones. Brain Res 263: 43–50
Murakoshi T, Otsuka M (1985) Respiratory reflexes in an isolated brainstem-lung preparation of the newborn rat: possible involvement of γ-aminobutyric acid and glycine. Neurosci Lett 62: 63–68
Murakoshi T, Suzue T, Tamai S (1985) A pharmacological study on respiratory rhythm in the isolated brainstem-spinal cord preparation of the newborn rat. Br J Pharmacol 86: 95–104
Onimaru H, Homma I (1987) Respiratory rhythm generator neurons in medulla of brainstem-spinal cord preparation from newborn rat. Brain Res 403: 380–384
Onimaru H, Arata A, Homma I (1987) Localization of respiratory rhythm-generating neurons in the medulla of brainstem-spinal cord preparations from newborn rats. Neurosci Lett 78: 151–155
Onimaru H, Arata A, Homma I (1988) Primary respiratory rhythm generator in the medulla of brainstem-spinal cord preparation from newborn rat. Brain Res 445: 314–324
Onimaru H, Arata A, Homma I (1988) Inhibitory synaptic inputs to respiratory rhythm generator in medulla isolated from newborn rats. J Physiol Soc Jpn 50: 584
Onimaru H, Arata A, Homma I (1989) Firing properties of respiratory rhythm generating neurons in the absence of synaptic transmission in rat medulla in vitro. Exp Brain Res 76: 530–536
Richter DW (1982) Generation and maintenance of the respiratory rhythm. J Exp Biol 100: 93–107
Rovainen CM (1983) Generation of respiratory activity by the lamprey brain exposed to picrotoxin and strychnine, and weak synaptic inhibition in motoneurons. Neuroscience 10: 875–882
Smith JC, Feldman JL (1986) Role of chloride-dependent synaptic inhibition in respiratory pattern generation. Studies in an in vitro mammalian brainstem-spinal cord preparation. Fed Proc 45: 518
Smith JC, Feldman JL (1987) In vitro brainstem-spinal cord preparations for study of motor systems for mammalian respiration and locomotion. J Neurosci Methods 21: 321–333
Smith JC, Feldman JL (1987) Central respiratory pattern generation studied in an in vitro mammalian brainstem-spinal cord preparation. In: Sieck GC, Gandevia SG, Cameron WE (eds) Respiratory muscles and their neuromotor control. Liss, New York, pp 27–36
Suzue T (1984) Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J Physiol (Lond) 354: 173–183
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Onimaru, H., Arata, A. & Homma, I. Inhibitory synaptic inputs to the respiratory rhythm generator in the medulla isolated from newborn rats. Pflugers Arch. 417, 425–432 (1990). https://doi.org/10.1007/BF00370663
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00370663