Abstract
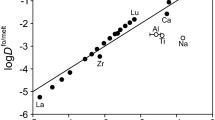
Partition coefficients for Cs, Ba, Sr, Ca, Mg, La, Sm, Lu, Mn, Ti, Cr, Ta, Zr, and P between immiscible basic and acidic liquids in the system K2O-Al2O3-FeO-SiO2 were experimentally determined at 1,180 °C and 1 atm. Phosphorus is most strongly enriched in the basic melt (by a factor of 10), followed by rare earth elements, Ta, Ca, Cr, Ti, Mn, Zr, Mg, Sr, and Ba (enriched by a factor of 1.5). Of the elements studied, only Cs is enriched in the acidic melt. The two-liquid partition coefficients of Zr, Ta, Sm, and Mn are constant for concentrations ranging from <0.1% to as high as 1 wt.-%, suggesting that Henry's law is applicable in silicate melts (at least for these elements) to concentrations well above typical trace element levels in rocks. The strong relative preference of many elements for the basic melt implies that the structural characteristics of basic melts more readily permit stable coordination of cations by oxygen. Partitioning of elements between crystal and liquid in a magma must therefore be influenced by the composition (and consequent structure) of the liquid.
Application of the two-liquid partition coefficients to possible occurrences of liquid immiscibility in magmas reveals that typical basalt-rhyolite associations are probably not generated by two-liquid phase separation. However, liquid immiscibility cannot be discounted as a possible origin for lamprophyric rocks containing felsic segregations.
Similar content being viewed by others
References
Nagasawa, H., Schnetzler, C.C.: Partitioning of rare earth, alkali, and alkaline earth elements between phenocrysts and acidic igneous magma. Geochim. Cosmochim. Acta 35, 935–968 (1971)
Burns, R.G., Fyfe, W.S.: Site of preference energy and selective uptake of transition metal ions from a magma. Science 144, 1001–1003 (1964)
Banno, S., Matsui, Y.: On the formulation of partition coefficients for trace element distribution between minerals and magma. Chem. Geol. 11, 1–15 (1973)
Hess, P.C.: Polymer model of silicate melts. Geochim. Cosmochim. Acta 35, 289–306 (1971)
Boon, J.A.: Mossbauer investigations in the system Na2O-FeO-SiO2. Chem. Geol. 7, 153–169 (1971)
Turner, W.H., Turner, J.A.: Ligand-field spectra and the structure of Ni in silicate glasses. J. Am. Ceram. Soc. 55, 201–207 (1972)
Boon, J.A., Fyfe, W.S.: The coordination number of ferrous ions in silicate glasses. Chem. Geol. 10, 287–298 (1972)
Roedder, E.: Low-temperature liquid immiscibility in the system K2O-FeO-Al2O3-SiO2. Am. Mineralogist 36, 282–286 (1951)
Schairer, J.F., Bowen, N.L.: The system K2O-Al2O3-SiO2. Am. J. Sci. 253, 681–746 (1955)
Bowen, N.L., Schairer, J.F.: The system, FeO-SiO2. Am. J. Sci., 5th series, 24, 177–213 (1932)
Finger, L.W., Hadidiacos, C.G.: Electron microprobe automation. Yb. Carnegie Inst. Wash. 71, 598–599 (1972)
Albee, A.L., Ray, L.: Correction factors for electron probe microanalysis of silicates, oxides, carbonates, phosphates, and sulfates. Anal. Chem. 42, 1408–1414 (1970)
Colby, J.W.: Quantitative microprobe analysis of thin insulating films. Advan. X-ray Anal. 11, 287–305 (1968)
Hess, P.C., Rutherford, M.J.: Element fractionation between immiscible melts [abstract]. In: Lunar science V, pp. 328–329. Houston: Lunar Science Institute 1974
Day, D.E., Rindone, G.E.: Properties of soda alumino-silicate glasses, III, Coordination of aluminum ions. J. Am. Ceram. Soc. 45, 579–581 (1962)
Verhoogen, J.: Distribution of titanium between silicates and oxides in igneous rocks. Am. J. Sci. 260, 211–220 (1962)
Doremus, R.H.: Glass science, 349 pp. New York: John Wiley and Sons 1973
Whittaker, E.J.W., Muntus, R.: Ionic radii for use in geochemistry. Geochim. Cosmochim. Acta 34, 945–956 (1970)
Glasser, F.P., Warshaw, I., Roy, R.: Liquid immiscibility in silicate systems. Phys. Chem. Glasses 1, 39–45 (1960)
Toop, G.W., Samis, C.S.: Activities of ions in silicate melts. Trans. Met. Soc. AIME 224, 878–887 (1962)
Levin, E.M., Block, S.: Structural interpretation of immiscibility in oxide systems: I, analysis and calculation of immiscibility. J. Am. Ceram. Soc. 40, 95–106 (1956)
Irvine, T.N.: Chromitite layers in stratiform intrusions. Yb. Carnegie Inst. Wash. 73, 300–316 (1974)
Roedder, E., Weiblen, P.W.: Petrology of silicate melt inclusions, Apollo 11 and Apollo 12 and terrestrial equivalents. In: Proc. Second Lunar Sci. Conf., Geochim. Cosmochim. Acta, Suppl. 2, Vol. 1, pp. 507–528. Cambridge: The M.I.T. Press 1971
Yoder, H.S., Jr.: Contemporaneous basaltic and rhyolitic magmas. Am. Mineralogist 58, 153–171 (1973)
Chayes, F.: Relative abundance of intermediate members of the oceanic basalt-trachyte association. J. Geophys. Res. 68, 1519–1534 (1963)
Haskin, L.A., Haskin, M.A., Frey, F.A., Wildeman, T.R.: Relative and absolute terrestrial abundances of the rare earths. In: Origin and distribution of the elements (L.H. Ahrens, ed.), pp. 889–912. Oxford: Pergamon Press 1968
Zielinski, R.A., Frey, F.A.: Gough Island: Evaluation of a frational crystallization model. Contrib. Mineral. Petrol. 29, 242–254 (1970)
Ewart, A., Taylor, S.R., Capp, A.C.: Geochemistry of the pantellerites of Mayor Island, New Zealand. Contrib. Mineral. Petrol. 17, 116–140 (1968)
Philpotts, A.R.: Immiscibility between feldspathic and gabbroic magmas. Nature Phys. Sci. 299, 107–109 (1971)
Ferguson, J., Currie, K.L.: Evidence of liquid immiscibility in alkaline ultrabasic dikes at Callander Bay, Ontario. J. Petrol. 12, 561–585 (1971)
Turner, F.J., Verhoogen, J.: Igneous and metamorphic petrology. 694 pp. New York: McGraw-Hill 1960
Bowen, N.L.: The evolution of the igneous rocks, 334 pp. Princeton University Press 1928
Nockolds, S.R.: Average chemical compositions of some igneous rocks. Geol. Soc. Am. Bull. 65, 1007–1032 (1954)
Cann, J.R.: Major element variations in ocean-floor basalts. Phil. Trans. Roy. Soc. London, Ser. A 268, 495–505 (1971)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Watson, E.B. Two-liquid partition coefficients: Experimental data and geochemical implications. Contr. Mineral. and Petrol. 56, 119–134 (1976). https://doi.org/10.1007/BF00375424
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00375424