Abstract
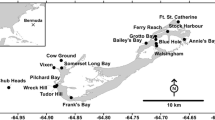
There has been an historical decline in the seagrass beds in Maho and Francis Bays, St. John, U.S. Virgin Islands: presently (1986) there are only five small seagrass beds in shallows water. These seagrass beds are highly disturbed by heavy boat usage and are intensively grazed by the green turtle Chelonia mydas L. Fifteen to 50 boats anchor each night in the bays: anchor scars cause a loss of up to 6.5 m2 d-1 or 1.8% yr-1 of the seagrass beds. Seagrasses regrew into such scars only minimally within a period of 7 mo. The size of the green turtle population was estimated at 50 subadults and their feeding behavior was determined by direct observation and radiotelemetry. The behavior of the green turtles differed from other observations published on the species. Here, the turtles grazed all available Thalassia testudinum, their preferred seagrass food, rather than creating discrete grazing scars, and spent all their waking hours (9 h per day) feeding. Areal productivity of T. testudinum leaves (33 to 97 mg dry wt m-2d-1) in the bays was at least an order of magnitude lower than published values or than the productivity of another, lessdisturbed seagrass bed on St. John, despite having comparable leaf-shoot density. Leaf shoots were stunted, fragile, achlorotic, and had only two leaves as opposed to the five leaves per shoot more typically seen. The green turtle population was near the estimated carrying capacity of T. testudinum, based on the standing crop and productivity of T. testudinum and the grazing rate of the turtles. The effect of disturbance of T. testudinum from boats and turtles was assessed by excluding these with emergent fences. Within 3 mo of protection, the areal and shoot-specific productivity of T. testudinum leaves as well as leaf size increased significantly compared to unprotected areas. Conservation efforts are recommended in Maho Bays and Francis because seagrass productivity is low, disturbance rates are higher than recovery rates, the turtles cannot increase further their feeding rate in order to compensate for such factors, and there are few alternate sources of T. testudinum on the north shore of St. John.
Similar content being viewed by others
Literature cited
Atkinson, M. J., Smith, S. V. (1983). C:N:P ratios of benthic marine plants. Limnol. Oceanogr. 28: 568–574
Bjorndal, K. A. (1980). Nutrition and grazing behavior of the green turtle Chelonia mydas. Mar. Biol. 56: 147–154
Bjorndal, K. A. (1981). The consequences of herbivory for the life history pattern of the Caribbean green turtle, Chelonia mydas. In: Bjorndal, K. A. (ed.) Biology and conservation of sea turtles. Smithsonian Institution, Washington, p. 111–116
Dawes, C. J., Brid, K., Durako, M., Goddard, R., Hoffman, W., McIntosh, R. (1979). Chemical fluctuations due to seasonal and cropping effects on an algal-seagrass community. Aquat. Bot. 6: 79–86
Dawes, C. J., Lawrence, J. M. (1979). Effects of blade removal on the proximate composition of the rhizome of the seagrass Thalassia testudinum Banks ex König. Aquat. Bot. 7: 255–266
Fuss, C. M., Kelly, J. A. (1969). Survival and growth of sea grasses transplanted under artificial conditions. Bull. mar. Sci. 19: 351–365
Greenway, M. (1974). The effects of cropping on the growth of Thalassia testudinum (König) in Jamaica. Aquaculture, Amsterdam 4: 199–206
Grime, J. P. (1977). Evidence for the existence of three primary strategies in the plants and its relevance to ecological and evolutionary theory. Am. Nat. 111: 1169–1194
Kelly, J. A., Fuss, C. M., Hall, J. R. (1971). The transplanting and survival of turtle grass, Thalassia testudinum, in Boca Ciega Bay, Florida. Fish. Bull. U.S. 69: 273–280
Kumpf, H. E., Randall, H. A. (1981). Charting the marine environments of St. John, U.S. Virgin Islands. Bull. mar. Sci. Gulf Caribb. 11: 543–551
Klug, M. J. (1980). Detritus-decomposition relationships. In: Phillips, R. C., McRoy, C. P. (eds.) Handbook of seagrass biology. Garland STPM Press, p. 225–245
McMillan, C. (1984). The condensed tannins (proanthocyanidins) in seagrasses. Aquat. Bot. 20: 351–357
McRoy, C. P., McMillan, C. (1977). Productivity and physiological ecology of seagrasses. In: McRoy, C. P., Helfferich, C. (eds.) Seagrass ecosystems: a scientific perspective. Marcel Dekker, New York, p. 53–88
Mortimer, J. A. (1981). Feeding ecology of sea turtles. In: Biology and conservation of sea turtles. Bjorndal, K. A. (ed.). Smithsonian Institution Press, Washington, D. C., p. 103–109
Ogden, J. C. (1976). Some aspects of herbivore-plant relationships in Caribbean reefs and seagrass beds. Aquat. Bot. 2: 103–116
Ogden, J. C. (1980). Faunal relationships in Caribbean seagrass beds. In: Phillips, R. C., McRoy, C. P. (eds.) Handbook of seagrass biology. Garland STPM Press, New York
Ogden, J. C., Robinson, L., Whitlock, K., Dagenhart, H., Cebula, R. (1983). Diel foraging patterns in juvenile green turtles (Chelonia mydas L.) in St. Croix, United States Virgin Islands. J. exp. mar. Biol. Ecol. 66: 199–205
Patriquin, D. G. (1973). Estimation of growth rate, production, and age of the marine angiosperm Thalassia testudinum König. Caribb. J. Sci. 13: 111–123
Patriquin, D. G. (1975). “Migration” of blowouts in seagrass beds at Barbados and Carriacou, West Indies, and its ecological and geological implications. Aquat. Bot. 1: 163–189
Phillips, R. C., LewisIII, R. L. (1983). Influence of environmental gradients on variations in leaf widths and transplant success in North American seagrasses. J. mar. technol. Soc. 17: 59–68
Rice, D. L. (1982). The detritus nitrogen problem: new observations and perspectives from organic geochemistry. Mar. Ecol. Prog. Ser. 9: 153–162
Rice, D. L., Tenore, K. R. (1981). Dynamics of carbon and nitrogen during the decomposition of detritus derived from estuarine macrophytes. Estuar. cstl Shelf Sci. 13: 681–690
Taylor, J. L., Saloman, C. H., Prest, K. W. (1973). Harvest and regrowth of turtle grass (Thalassia testudinum) in Tampa Bay, Florida. Fish. Bull. U.S. 71: 145–148
Thayer, G. W., Bjorndal, K. A., Ogden, J. C., Williams, S. L., Zieman, J. C. (1985). Role of large herbiovores in seagrass communities. Estuaries 7: 351–376
Thayer, G. W., Engel, D. W., Bjorndal, K. A. (1982). Evidence for short-circuiting of the detritus cycle of seagrass beds by the green turtle, Chelonia mydas L. J. exp. mar. Biol. Ecol. 62: 173–183
Thayer, G. W., Wolfe, D. A., Williams, R. B. (1975). The impact of man on seagrass systems. Am. Scient 63: 289–296
Tighe, S. A. (1981). Grazing of seagrasses by the green turtle (Chelonia mydas) in St. Croix, U.S.V.I. MS thesis, Fairleigh Dickinson University, Rutherford, New Jersey
Tomlinson, P. B. (1974). Vegetative morphology and meristem dependence — the foundation of productivity in seagrasses. Aquaculture, Amsterdam 4: 107–130
Williams, S. L., Breda, V. A., Anderson, T. W., Nyden, B. B. (1985). Growth and sediment disturbances of Caulerpa spp. (Chlorophyta) in a submarine canyon. Mar. Ecol. Prog. Ser. 21: 275–281
Zieman, J. C. (1974). Methods for the study of the growth and production of turtle grass, Thalassia testudinum König. Aquaculture, Amsterdam 4: 139–142
Zieman, J. C. (1976). The ecological effects of physical damage from motor boats on turtle grass beds in southern Florida. Aquat. Bot. 2: 127–139
Zieman, J. C., Iverson, R. L., Ogden, J. C. (1984). Herbivory effects on Thalassia testudinum leaf growth and nitrogen content. Mar. Ecol. Prog. Ser. 15: 151–158
Zieman, J. C., Wetzel, R. G. (1980). Productivity in seagrasses: methods and rates. In: Phillips, R. C., McRoy, C. P. (eds.) A handbook of seagrass biology. Garland STPM Press, New York, p. 87–116
Author information
Authors and Affiliations
Additional information
Communicated by: P. C. Schroeder, Pullman
Contribution No. 175 from West Indies Laboratory, Teague Bay, Christiansted, St. Croix, U.S. Virgin Islands 00820, USA
Rights and permissions
About this article
Cite this article
Williams, S.L. Thalassia testudinum productivity and grazing by green turtles in a highly disturbed seagrass bed. Marine Biology 98, 447–455 (1988). https://doi.org/10.1007/BF00391121
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00391121