Abstract
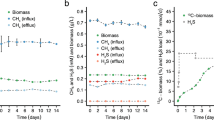
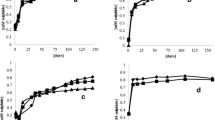
Desulfovibrio vulgaris (Marburg) and Methanobrevibacter arboriphilus (AZ) are anaerobic sewage sludge bacteria which grow on H2 plus sulfate and H2 plus CO2 as sole energy sources, respectively. Their apparent Ks values for H2 were determined and found to be approximately 1 μM for the sulfate reducing bacterium and 6 μM for the methanogenic bacterium. In mixed cell suspensions of the two bacteria (adjusted to equal V max) the rate of H2 consumption by D. vulgaris was five times that of M. arboriphilus, when the hydrogen supply was rate limiting. The apparent inhibition of methanogenesis was of the same order as expected from the different Ks values for H2. Difference in substrate affinities can thus account for the inhibition of methanogenesis from H2 and CO2 in sulfate rich environments, where the H2 concentration is well below 5 μM.
Similar content being viewed by others
References
Abram JW, Nedwell DB (1978a) Inhibition of methanogenesis by sulfate-reducing bacteria competing for transferred hydrogen. Arch Microbiol 117:89–92
Abram JW, Nedwell DB (1978b) Hydrogen as a substrate for mathyanogenesis and sulfate reduction in anaerobic saltmarsh sediments. Arch Microbiol 117:93–97
Badziong W, Thauer RK, Zeikus JG (1978) Isolation and characterization of Desulfovibrio growing on hydrogen plus sulfate as sole energy source. Arch Microbiol 116:41–49
Badziong W, Thauer RK (1978) Growth yields and growth rates of Desulfovibrio vulgaris (Marburg) growing on hydrogen plus sulfate and hydrogen plus thiosulfate as the sole energy sources. Arch Microbiol 117:209–214
Badziong W, Thauer PK (1980) Vectorial electron transport in Desulfovibrio vulgaris (Marburg) growing on hydrogen plus sulfate as sole energy source. Arch Microbiol 125:167–174
Bell GR, Legall J, Peck HD (1974) Evidence for the periplasmic location of hydrogenase in Desulfovibrio gigas. J. Bacteriol 120:994–997
Brandis A, Thauer RK (1981) Growth of Desulfovibrio species on hydrogen an sulphate as sole energy source. J Gen Microbiol 126:249–252
Bryant MP, Campbell LL, Reddy CA, Crabill MR (1977) Growth of Desulfovibrio in lactate or ethanol media low in sulfate in association with H2-utilizing methanogenic bacteria. Appl Environ Microbiol 33:1162–1169
Cappenberg TE (1974) Interrelationships between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh-water lake. I. Field observations. Antonie van Leeuwenhoek J Microbiol Serol 40:285–295
Fersht A (1977) Enzyme structure and mechanism. Freeman and Co, Reading, San Francisco
Fuchs G, Moll J, Scherer P, Thauer R (1979) Activity, acceptor specificity and function of hydrogenase in Methanobacterium thermoautotrophicum. In: HG Schlegel, K Schneider (eds) Hydrogenase: Their catalytic activity, structure and function. Goltze, Göttingen, pp 83–92
Hungate RE (1967) Hydrogen as an intermediate in the rumen fermentation. Arch Microbiol 59:158–164
Hungate RE, Smith W, Bauchop T, Yu J, Rabinowitz JC (1970) Formate as an intermediate in the bovine rumen fermentation. J Bacteriol 102:389–397
Jones LW, Bishop NI (1976) Simultaneous measurement of oxygen and hydrogen exchange from the blue-green alga Anabaena. Plant Physiol 57:659–665
Jørgensen BB (1977) The sulphur cycle of a coastal marine sediment (Limfjorden, Denmark). Limmol Oceanogr 22:814–832
Jørgensen BB (1980) Mineralization and the bacterial cycling of carbon, nitrogen and sulfur in marine sediments. In: DC Ellwood, JN Hedger, MJ Latham, JM Lynch, JH Slater (eds) Contemporary microbial ecology. Academic Press, London New York Toronto Sydney San Francisco, pp 239–351
Kaspar HF, Wuhrmann K (1978) Kinetic parameters and relative turnovers of some important catabolic reactions in digesting sludge. Appl Environ Microbiol 36:1–7
Kristjansson JK, Hollocher TC (1980) First practical assay for soluble nitrous oxide reductase of denitrifying bacteria and a partial kinetic characterization. J Biol Chem 255:704–707
Martens CS, Berner RA (1974) Methane production in the interstitial waters of sulfate-depleted marine sediments. Science 185:1167–1169
McCarty PL (1972) Energetics of organic matter degradation. In: R Mitchel (ed) Water pollution microbiology. Wiley-Interscience, New York, pp 91–113
McInerney JM, Bryant MP (1981) Anaerobic degradation of lactate by synthrophic association of Methanosarcina barkeri and Desulfovibrio species and effect of H2 on acetate degradation. Appl Environ Microbiol 41:346–354
Mountfort DO, Asher RA (1979) Effect of inorganic sulfide on the growth and metabolism of Methanosarcina barkeri strain DM. Appl Environ Microbiol 37:670–675
Mountfort DO, Asher RA, Mays EL, Tiedje JE (1980) Carbon and electron flow in mud and sandflat intertidal sedimengs of Delaware inlet, Nelson, New Zealand. Appl Environ Microbiol 39:686–694
Oremland RS, Taylor BF (1978) Sulfate reduction and methanogenesis in marine sediments. Geochim Cosmochim Acta 42:209–214
Robinson JA, Strayer RF, Tiedje JM (1981) Method for measuring dissolved hydrogen in anaerobic ecosystems: Application to the rumen. Appl Environ Microbiol 41:545–548
Scherer P, Sahm H (1981) Influence of sulphur-containing compounds on the growth of Methanosarcina barkeri in a defined medium. Eur J Appl Microbiol Biotechnol 12:28–35
Schönheit P, Moll J, Thauer RK (1980) Growth parameters (Ks, 282-1, Ys) of Methanobacterium thermoautotrophicum. Arch Microbiol 127:59–65
Strayer RF, Tiedje JM (1978) Kinetic parameters of the conversion of methane precursors to methane in hypereutrophic lake sediment. Appl Environ Microbiol 36:330–340
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Veldkamp H, Jannasch HW (1972) Mixed culture studies with the chemostat. J Appl Chem Biotechnol 22:105–123
Wang R, Healey FP, Myers J (1971) Amperometric measurement of hydrogen evolution in Clamydomonas. Plant Physiol 48:108–110
Wellinger A, Wuhrmann K (1977) Influence of sulfide compounds on the metabolism of Methanobacterium strain ATZ. Arch Microbiol 115:13–17
Winfrey MR, Zeikus JG (1977) Effect of sulfate on carbon and electron flow during microbiol methanogenesis in freshwater sediments. Appl Environ Microbiol 33:275–281
Zehnder AJB, Wuhrmann K (1977) Physiology of a Methanobacterium strain AZ. Arch Microbiol 111:199–205
Zehnder AJB (1978) Ecology of methane formation. In: R Mitchell (ed) Water pollution microbiology, Vol 2. John Wiley and Sons Inc, New York, pp 349–376
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kristjansson, J.K., Schönheit, P. & Thauer, R.K. Different Ks values for hydrogen of methanogenic bacteria and sulfate reducing bacteria: An explanation for the apparent inhibition of methanogenesis by sulfate. Arch. Microbiol. 131, 278–282 (1982). https://doi.org/10.1007/BF00405893
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00405893