Abstract
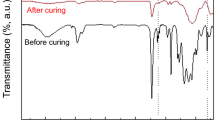
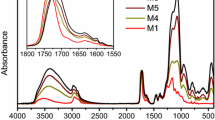
Novel abrasion resistant coating materials prepared by the sol-gel method have been developed and applied on the polymeric substrates bisphenol-A polycarbonate and diallyl diglycol carbonate resin (CR-39). These coatings are inorganic/organic hybrid network materials synthesized from 3-isocyanatopropyltriethoxysilane functionalized organics and metal alkoxide. The organic components are 3,3′-iminobispropylamine (IMPA), resorcinol (RSOL), diethylenetriamine (DETA), poly(ethyleneimine) (PEI), glycerol and a series of diols. The metal alkoxides are tetraethoxysilane (TEOS) and tetramethoxysilane (TMOS). These materials are spin coated onto bisphenol-A polycarbonate and CR-39 sheets and thermally cured to obtain a transparent coating of a few microns in thickness. Following the curing, the abrasion resistance is measured and compared with an uncoated control. It was found that the abrasion resistance of inorganic/organic hybrid coatings in the neat form or containing metal alkoxide can be very effective to improve the abrasion resistance of polymeric substrates. The adhesion tests show that the adhesion between coating and substrate can be greatly improved by treating the polymeric substrate surface with a primer solution of isopropanol containing 3-aminopropyltriethoxysilane (3-APS). The interaction between 3-APS and the polycarbonate surface was investigated by a molecular dynamics simulation. The results strongly suggest that the hydrogen bonding between the amino group of the 3-APS and ester group in the polycarbonate backbone are sufficiently strong to influence the orientation of the primer molecules. The abrasion resistance of these new coating systems is discussed in light of the structure of the organic components. All of these results show that these coating materials have excellent abrasion resistance and have potential applications as coating materials for lenses and other polymeric products.
Similar content being viewed by others
References
J. Kasi, JPn. Kokai Tokkyo Koho, JP6335, 633 (88,35,633).
R. Nass, E. Appac, W. Glaubitt, and H. Schmidt, J. Non-Cryst. Solids 121, 370 (1990).
H. Schmidt and H. Walter, J. Non-Cryst. Solids 121, 428 (1990).
H. Schmidt, Mat. Res. Soc. Symp. Proc. 32, 327 (1984).
G.L. Wilkes, B. Orler, and H.H. Huang, Polymer Preprints 26(2), 300 (1985).
H. Huang and G.L. Wilkes, Polymer Bulletin 18, 455 (1987).
H. Huang, R.H. Glaser, and G.L. Wilkes, ACS Symp. Ser. 360, 354 (1988).
H. Huang and G.L. Wilkes, Polymer 30, 2001 (1989).
Y. Hu and J.D. Mackenzie, Mat. Res. Soc. Symp. Proc. 271, 681 (1992).
Y. Hu and J.D. Mackenzie, J. Mat. Sci. 27, 4415 (1992).
J.D. Machenzie, Y.J. Chung, and Y. Hu, J. Non-Cryst. Solids, 147 & 148, 271 (1992).
J.E. Mark and S.-J. Pan, Makromol. Chem. Rapid Commun. 3, 681 (1982).
J.E. Mark, C.-Y. Jiang and M.-Y. Tang, Macromolecules 17, 2613 (1984).
J.E. Mark, J. Appl. Polym. Sci., Appl. Polym. Symp. 50, 273 (1992).
J.E. Mark, Frontier of Polym. & Advanced Mater., edited by P.N. Prasad (Plenum Press, New York, 1994), p. 403.
B.M. Novak, Advanced Materials 5, 422 (1993).
H. Schmidt, Mat. Res. Soc. Symp. Proc. 171, 3 (1990).
B.M. Novak and R.H. Grubbs, J. Am. Chem. Soc. 110, 7542 (1988).
S. Wang, Z. Ahmad, and J.E. Mark, Macromol. Reports A31, 411 (1994).
J.L.W. Noell, G.L. Wilkes, D.K. Mohauty, and J.E. McGrath, J. Appl. Polym. Sci. 40, 1177 (1990).
C. Betrabet and G.L. Wilkes, Polymer Preprints 32(2), 286 (1992).
B. Tamami, C. Betrabet, and G.L. Wilkes, Polymer Bulletin 30, 39 (1993).
B. Tamami, C. Betrabet, and G.L. Wilkes, Polymer Bulletin 30, 393 (1993).
B. Wang and G.L. Wilkes, J. Macromol. Sci-Pure Appl. Chem. A31, 249 (1994).
B. Wang, G.L. Wilkes, C.D. Smith, and J.E. McGrath, Polymer Commun. 32, 400 (1991).
J. Wen and G.L. Wilkes, J. Inorganic & Organometallic Polymers 5, 000 (1995).
C. Betrabet and G.L. Wilkes, J. Inorganic & Organometallic Polymers 4, 343 (1994).
M. El-Shabsy, Period. Polytech. Electr. Eng. 25, 283 (1981).
Y. Zhan and W.L. Mattice, Macromolecules 27, 7056 1994.
A. Selwood, Wear 4, 311 (1961).
W.D. Bascom, Eng. Mater. Handbook (ASM International EDS., 1990) V3, p. 254.
E.P. Plueddemann, Adhesion Aspects of Polymeric Coatings, edited by K.L. Mittal (Plenum Press, New York, 1983), p. 319.
S.L. Mayo, B.D. Olafson, and W.A. Goddard III, J. Phys. Chem. 94, 8897 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wen, J., Vasudevan, V.J. & Wilkes, G.L. Abrasion resistant inorganic/organic coating materials prepared by the sol-gel method. J Sol-Gel Sci Technol 5, 115–126 (1995). https://doi.org/10.1007/BF00487727
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00487727