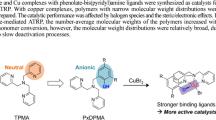
Abstract
A new cluster-type monomer [Fe3O(OCOCH=CH2)6]OH · 3H2O was synthesized by the reaction of acrylic acid with freshly prepared iron hydroxide. The monomer was characterized by various physicochemical methods. It has been shown that the acid residues are bound to the metal atoms through carboxyl groups in a bridge-like or bidentate fashion, and the double bond does not participate in the coordination. The mechanism of thermal fragmentation of the [Fe3O(OCOCH=CH2)6]+ cluster was studied. It was demonstrated that the radical polymerization of this monomer involved bidentate acrylate groups. No reduction of Fe3+ during the polymerization was observed.
Similar content being viewed by others
References
Yu. M. Shul'ga, O. S. Roshchupkina, G. I. Dzhardimalieva, and A. D. Pomogailo,Izv. Akad. Nauk, Ser. Khim., 1993, 1565 [Russ. Chem. Bull., 1993,42, 1498 (Engl. Transl.)].
A. D. Pomogailo and V. S. Savostyanov,Metallosoderzhashchie monomery i polimery na ikh osnove [Metal-containing Monomers and Polymers Based on Them], Khimia, Moscow, 1988, 384 (in Russian).
R. A. Lidin, L. L. Andreeva, and V. A. Molochko,Spravochnik po neorganicheskoi khimii [Handbook of Inorganic Chemistry], Khimia, Moscow, 1987, 320 (in Russian).
G. I. Dzhardimalieva, A. D. Pomogailo, V. I. Ponomarev, L. O. Atovmyan, Yu. M. Shul'ga, and A. G. Starichkov,Izv. Akad. Nauk. SSSR, Ser. Khim., 1988, 1525 [Bull. Acad. Sci. USSR, Div. Chem. Sci., 1988,37, 1346 (Engl. Transl.)].
USSR Pat. 1681340,Byul. Izobret., 1991, 36.
I. V. Chernushevich, Ph. D. (Phys.-Mat. Sci.) Thesis, Branch of the Institute of Energetic Problems of Chemical Physics of the RAS, Chernogolovka, 1991, 246 (in Russian).
B. N. Figgis and G. B. Robertson,Nature, 1965,205, 694.
F. A. Cotton and G. Wilkinson,Advanced inorganic chemistry, Part 3, Wiley-Interscience, New York, 1967.
G. J. Long, W. N. Robinson, W. P. Tappmeyer, and D. L. Bridges,J. Chem. Soc., Dalton Trans., 1973, 573.
K. Nakamoto,Infrared and Roman Spectra of Inorganic and Coordination Compounds, Wiley, New York, 1986.
K. Nakanishi,Infrared Absorption Spectroscopy, Holden-Day, Inc., San Francisco, 1962.
Author information
Authors and Affiliations
Additional information
For part 32 see Ref. 1.
Translated fromIzvestiya Akademii Nauk. Seriya Khimicheskaya, No. 10, pp. 1739–1743, October, 1993.
Rights and permissions
About this article
Cite this article
Shul'ga, Y.M., Roshchupkina, O.S., Dzhardimalieva, G.I. et al. Preparation and reactivity of metal-containing monomers. Russ Chem Bull 42, 1661–1665 (1993). https://doi.org/10.1007/BF00697035
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00697035