Abstract
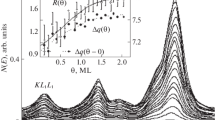
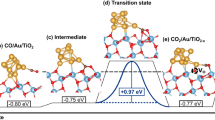
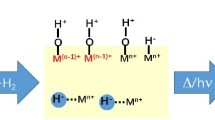
Sub-monolayer quantities of metal oxides are found to influence CO hydrogenation, CO2 hydrogenation, acetone hydrogenation, ethylene hydroformylation, ethylene hydrogenation, and ethane hydrogenolysis over Rh foils. The metal oxides investigated include AlOx, TiOx, VOx, FeOx, ZrOx, NbOx, TaOx, and WOx. Only those reactions involving the hydrogenation of C-O bonds are enhanced by the oxide overlayers. The coverage at which maximum rate enhancement occurs is approximately 0.5 ML for each oxide promoter. Titanium, niobium, and tantalum oxides are the most effective promoters. XPS measurements after reaction show that of the oxides studied titanium, niobium, and tantalum oxide overlayers are stable in the highest oxidation states. The trend in promotion effectiveness is attributed to the direct relationship between oxidation state and Lewis acidity. For the oxide promoters, bonding at the metal oxide/metal interface between the O-end of adsorbed CO and the Lewis acidic oxide is postulated to facilitate C-O bond dissociation and subsequent hydrogenation.
Similar content being viewed by others
References
S.J. Tauster, Acc. Chem. Res. 20 (1987) 389.
A.T. Bell, in:Catalyst Design — Progress and Perspectives, ed. L.L. Hegedus (Wiley, New York, 1987).
R. Burch, in:Hydrogen Effects in Catalysis, eds. Z. Paal and P.G. Menon (Dekker, New York, 1988).
G.L. Haller and D.E. Resasco, Adv. Catal. 36 (1989) 173.
M.A. Vannice, Catal. Today 12 (1992) 255.
I. Mochida, I. Nonugide, H. Ishibashi and H. Fujitsu, J. Catal. 110 (1988) 159.
T. Koerts, W.J.J. Welters and R.A. van Santen, J. Catal. 134 (1992) 1.
W.M.H. Sachtler and M.J. Ichikawa, J. Phys. Chem. 90 (1986) 4752.
T. Iizuka, Y. Tanaka and K. Tanabe, J. Catal. 76 (1982) 1.
T. Iizuka, Y. Tanaka and K. Tanabe, J. Mol. Catal. 17 (1982) 381.
A. Trovarelli, C. Mustazza, G. Dolcetti, J. Kaspar and M. Graziani, Appl. Catal. 65 (1990).
P. Johnston, R.W. Joyner, P.D.A. Pudney, E.S. Shapiro and B.P. Williams, Faraday Discussions Chem. Soc. 89 (1990) 91.
J.P. Hindermann, A. Kiennemann and S. Tazkritt, in:Structure and Reactivity of Surfaces, eds. C. Morterra, A. Zecchina and G. Costa (Elsevier, Amsterdam, 1989).
N.T. Pande and A.T. Bell, J. Catal. 98 (1986) 577.
R. Burch and A.R. Flambard, J. Catal. 86 (1982) 384.
W.M.H. Sachtler, D.F. Shriver, W.B. Hollenberg and A.F. Lang, J. Catal. 92 (1985) 429.
M.E. Levin, M. Salmeron, A.T. Bell and G.A. Somorjai, J. Catal. 106 (1987) 401.
M.E. Levin, M. Salmeron, A.T. Bell and G.A. Somorjai, Faraday Trans. 183 (1987) 2061.
M.E. Levin, M. Salmeron, A.T. Bell and G.A. Somorjai, Surf. Sci. 195 (1988) 429.
A.B. Boffa, A.T. Bell and G.A. Somorjai, J. Catal. 139 (1993) 602.
A.B. Boffa, A.T. Bell and G.A. Somorjai, J. Catal., in press.
K.J. Williams, A.B. Boffa, M. Salmeron, A.T. Bell and G.A. Somorjai, Catal. Lett. 5 (1990) 385.
K.J. Williams, A.B. Boffa, M. Salmeron, A.T. Bell and G.A. Somorjai, Catal. Lett. 9 (1991) 41.
K.J. Williams, A.B. Boffa, M. Salmeron, A.T. Bell and G.A. Somorjai, Catal. Lett. 11 (1991) 77.
K.J. Williams, M. Salmeron, A.T. Bell and G.A. Somorjai, Catal. Lett. 1 (1988) 331.
H.C. Wang, D.F. Ogletree and M. Salmeron, J. Vac. Sci. Tech. B 9 (1992) 853.
K.J. Williams, M. Salmeron, A.T. Bell and G.A. Somorjai, Surf. Sci. 204 (1988) L745.
K.J. Williams, PhD Thesis, Department of Chemical Engineering, University of California at Berkeley, USA (1990).
A.B. Boffa, PhD Thesis, Department of Chemistry, University California at Berkeley, USA (1994).
K.I. Tanaka and A. Ozaki, J. Catal. 8 (1967) 1.
R.T. Sanderson,Chemical Periodicity (Reinhold, New York, 1960).
C.P. Horwitz and D.F. Shiver, Adv. Organomet. Chem. 23 (1984) 219.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Boffa, A.B., Lin, C., Bell, A.T. et al. Lewis acidity as an explanation for oxide promotion of metals: implications of its importance and limits for catalytic reactions. Catal Lett 27, 243–249 (1994). https://doi.org/10.1007/BF00813909
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00813909