Abstract
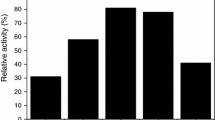
Two sets of WO3/SiO2 catalysts were prepared from (NH4 6H2W12O40 (aqueous method) and W(η3-C3H5)4 (non-aqueous method). The molecular structures and dispersions of the surface tungsten oxide species for the WO3/SiO2 catalysts under ambient and in situ dehydrated conditions were investigated by Raman spectroscopy. The samples prepared from (NH4)6H2W12O40 (aqueous method) exhibit very strong Raman features due to the presence of crystalline WO3 and the samples prepared from W(η3-C3H5)4 (non-aqueous method) do not possess crystalline WO3. These results suggest that the preparation method exerts an influence on the dispersion of the surface tungsten oxide species on SiO2. The surface tungsten oxide species under ambient conditions possess polytungstate clusters, W12O 12−42 , on the silica support. Upon dehydration at elevated temperatures, the hydrated polytungstate clusters decompose and interact with the silica support via the formation of isolated, octahedrally coordinated tungsten oxide species.
Similar content being viewed by others
References
S.R. Stampfl, Y. Chen, J.A. Dumesic, C. Niu and C.G. Hill Jr., J. Catal. 105 (1987) 445.
R.D. Roark, S.D. Kohler, J.G. Ekerdt, D.S. Kim and I.E. Wachs, Catal. Lett. 16 (1992) 77.
Y. Iwasawa, in:Advances in Catalysis, Vol. 35 (Academic Press, New York, 1987) p. 265.
Y.I. Yermakov, Catal. Rev. Sci. Eng. 13 (1976) 77.
J.P. Candlin and H. Thomas, Adv. Chem. Ser. 132 (1974) 212.
L. Rodrigo, K. Marcinkowska, A. Adnot, P.C. Roberge, S. Kaliaguine, J.M. Stencil, L.E. Makovsky and J.R. Diehl, J. Phys. Chem. 90 (1986) 2690.
C.C. Williams, J.G. Ekerdt, J.M. Jehng, F.D. Hardcastle, A.M. Turek and I.E. Wachs, J. Phys. Chem. 95 (1991) 8781.
C.C. Williams and J.G. Eckerdt, J. Catal. 141 (1993) 430.
N. Spencer, J. Catal. 109 (1988) 187.
D.S. Kim and I.E. Wachs, J. Catal. 141 (1993) 419.
F.D. Hardcastle and I.E. Wachs, J. Mol. Catal. 46 (1988) 173.
S.S. Chan, I.E. Wachs and L.L. Murrell, J. Catal. 90 (1984) 150.
W.P. Griffith and P.J.B.J. Lesniak, J. Chem. Soc. A (1969) 1066.
C.F. Baes and R.E. Mesmer, in:Hydrolysis of Cations (Wiley, New York, 1986).
G. Deo and I.E. Wachs, J.Phys. Chem. 95 (1991) 5889.
D.S. Kim and I.E. Wachs, J. Catal. 136 (1992) 539.
S.D. Kohler, J.G. Ekerdt, D.S. Kim and I.E. Wachs, Catal. Lett. 16 (1992) 231.
G.A. Parks, Chem. Rev. 65 (1965) 177.
F.D. Hardcastle, PhD Thesis, Lehigh University, USA (1990); F.D. Hardcastle and I.E. Wachs, J. Raman Spectry., in press.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kim, D.S., Ostromecki, M., Wachs, I.E. et al. Preparation and characterization of WO3/SiO2 catalysts. Catal Lett 33, 209–215 (1995). https://doi.org/10.1007/BF00814225
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00814225