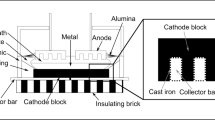
Abstract
A study of the effects of anode-cathode distance (ACD) on the cell potential and electrical bath resistivity in a laboratory scale cell that utilizes a sloping TiB2 composite cathode shows an increasing bath resistivity as the ACD decreases, due to a simultaneously increasing gaseous phase fraction in the electrolyte. The effective bath resistivity was found to increase rapidly after ACD=10 mm and was proportional to the inverse of ACD within this low ACD range studied (2–10 mm). Addition of NaCl to the electrolyte (5.5 wt%) lowered the electrical resistivity by increasing the conductivity of the electrolyte, and at low ACD it also lowered the bubble contribution to the bath resistivity, most likely due to changes in the hydrodynamic properties of the system. Lowering the cryolite ratio (i.e. molar ratio of NaF to AlF3) resulted in a higher electrical resistivity in the electrolyte by decreasing the conductivity of the melt, as well as by changing the hydrodynamic properties of the melt, leading to an increased bubble contribution to bath resistivity at low ACD. An increase in temperature resulted in a reduced bubble contribution at low ACD in a similar manner as the NaCl addition. From these results, it is clear that for a commericial process, reduction of the ACD and optimization of process conditions to reduce the effect of bubbles should allow significant savings in the energy requirements of the Hall-Heroult process.
Similar content being viewed by others
References
K. Billehaug and H. A. Øye,Aluminium 56 (1980) 642, 713.
K. Grjotheim, C. Krohn, M. Malinovsky, K. Matiasovsky and J. Thonstad, ‘Aluminium Electrolysis’, Aluminium Verlag GmbH, Dusseldorf (1982).
K. W. Tucker, J. T. Gee, J. R. Shaner, L. A. Joo, A. T. Tabereaux, D. V. Stewart and N. E. Richards,Light Metals (1987) 345.
R. C. Doward,J. Appl. Electrochem. 13 (1983) 569.
K. Grjotheim and B. J. Welch, ‘Aluminium Smelter Technology’, Aluminium Verlag GmbH, Dusseldorf (1980).
W. E. Haupin and W. B. Frank, in ‘Comprehensive Treatise of Electrochemistry’ (edited by J. O'M. Bockris et al.). Plenum, New York (1981) Vol. 2, Ch. 5, p. 301.
JANAF Thermochemical Tables (1965).
J. Thonstad,Electrochim. Acta 15 (1970) 1569.
R. Piontelli, B. Mazza and P. Pedeferri,Electrochim. Acta 10 (1965) 1117.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kasherman, D., Skyllas-Kazacos, M. Effects of anode-cathode distance on the cell potential and electrical bath resistivity in an aluminium electrolysis cell with a sloping TiB2 composite cathode. J Appl Electrochem 18, 863–868 (1988). https://doi.org/10.1007/BF01016043
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01016043