Abstract
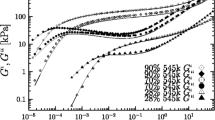
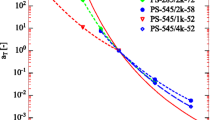
The solution viscosity of narrow molecular weight distribution polystyrene samples dissolved in toluene and trans-decalin was investigated. The effect of polymer concentration, molecular weight and shear rate on viscosity was determined. The molecular weights lay between 5 ⋅ 104 and 24 ⋅ 106 and the concentrations covered a range of values below and above the critical valuec *, at which the macromolecular coils begin to overlap. Flow curves were generated for the solutions studied by plotting logη versus log\(\dot \gamma \). Different molecular weights were found to have the same viscosity in the non-Newtonian region of the flow curves and follow a straight line with a slope of − 0.83. A plot of logη 0 versus logM w for 3 wt-% polystyrene in toluene showed a slope of approximately 3.4 in the high molecular weight regime. Increasing the shear rate resulted in a viscosity that was independent of molecular weight. The sloped (logη)/d (logM w ) was found to be zero for molecular weights at which the corresponding viscosities lay on the straight line in the power-law region.
On the basis of a relation betweenη sp and the dimensionless productc · [η], simple three-term equations were developed for polystyrene in toluene andt-decalin to correlate the zero-shear viscosity with the concentration and molecular weight. These are valid over a wide concentration range, but they are restricted to molar masses greater than approximately 20000. In the limit of high molecular weights the exponent ofM w in the dominant term in the equations for both solvents is close to the value 3.4. That is, the correlation betweenη sp andc · [η] results in a sloped(logη sp)/d(logc · [η]) of approximately 3.4/a at high values ofc · [η] wherea is the Mark-Houwink constant. This slope of 3.4/a is also the power ofc in the plot ofη 0 versusc at high concentrations.
Similar content being viewed by others
Abbreviations
- a :
-
Mark-Houwink constant
- B 1,B 2,B n :
-
constants
- c :
-
concentration (g · cm−3)
- c * :
-
critical concentration (g · cm−3)
- K, K′ :
-
constants
- K H :
-
Huggins constant
- M :
-
molecular weight
- M c :
-
critical molecular weight
- M n :
-
number-average molecular weight
- M w :
-
weight-average molecular weight
- n :
-
sloped(logη sp)/d (logc · [η]) at highc · [η]
- PS :
-
polystyrene
- T :
-
temperature (K)
- \(\dot \gamma \) :
-
shear rate (s−1)
- \(\dot \gamma \) :
-
critical shear rate (s−1)
- η :
-
viscosity (Pa · s)
- η 0 :
-
zero-shear viscosity (Pa · s)
- η s :
-
solvent viscosity (Pa · s)
- η sp :
-
specific viscosity
- [η]:
-
intrinsic viscosity (cm3 · g−1)
- η′ :
-
dynamic viscosity (Pa · s)
- |η *|:
-
complex dynamic viscosity (Pa · s)
- ω :
-
angular frequency (rad/s)
- ϱ :
-
density of polymer solution (g · cm−3)
- σ 12 :
-
shear stress (Pa)
References
Kniewske R, Kulicke W-M (1983) Makromol Chem 184:2173
Kulicke W-M, Kniewske R, Klein J (1982) Progr Polym Sci 8:373
Graessley WW (1974) Adv Polym Sci 16:49
Doi M (1981) ACS Polymer Preprints 22/1:100
Masuda T, Kitagawa K, Onogi S (1970) Polymer J (Japan) 1:418
Kulicke W-M, Klare J (1980) Angew Makromol Chem 84:67
Casale A, Moroni A, Civardi E (1976) Angew Makromol Chem 53:1
Berry GC, Fox TG (1968) Adv Polym Sci 5:216–357
Casale A, Porter RS, Johnson JF (1971) J Macromol Sci-Rvs Macromol Chem C 5(2):387
Schurz J (1975) Rheol Acta 14:293
Frind H, Schramek W (1955) Makromol Chem 17:1
Schramek W (1955) Makromol Chem 17:19
Weissberg SG, Simha R, Rothman S (1951) J Research NBS 47:298
Onogi S, Kimura S, Kato T, Masuda T, Miyanaga N (1966) J Polym Sci C 15:381
Hayahara T, Takao S (1968) Kolloid-Z Z Polym 225:106
Hoftyzer PJ, van Krevelen DW (1976) Angew. Makromol Chem 56:1
Rudin A, Strathdee GB, Edey WB (1973) J Appl Polym Sci 17:3085
Rudin A, Strathdee GB (1974) J Paint Technol 46:33
Zakin JL, Wu R, Luh H, Mayhan KG (1976) J Polym Sci Polym Phys Ed 14:299
Abdel-Alim AH, Balke ST, Hamielec AE (1973) J Appl Polym Sci 17:1431
Attané P, LeRoy P, Picard JM, Turrel G (1981) J Non-Newtonian Fluid Mech 9:13
Ballauf M (1981) Thesis, Mainz
Kulicke W-M, Porter RS (1981) J Polym Sci Polym Phys Ed 19:1173
Stratton RA (1966) J Colloid Interface Sci 22:517
Onogi S, Kato H, Ueki S, Ibaragi T (1966) J Polym Sci C 15:481
Onogi S, Masuda T, Ibaragi T (1968) Kolloid-Z Z Polym 222:110
Nielsen LE (1977) Polymer Rheology. Dekker M INC, New York, p 71
Vinogradov GV, Malkin AYa (1980) Rheology of Polymers, Springer-Verlag, Berlin, p 178 and p 185
Schurz J (1974) Struktur-Rheologie, Berliner Union Stuttgart, p 73
Porter RS, Johnson JF (1963) Trans Soc Rheology 7:241
Han ChD (1976) Rheology in Polymer Processing, Academic Press, New York, p 71
Bird RB, Armstrong RC, Hassager O (1977) Dynamics of Polymeric Liquids, J. Wiley & Sons, New York, vol 1, p 145
Klein J, Kulicke W-M (1976) Rheol Acta 15:558
Chou LY, Zakin JL (1967) J Colloid Interface Sci 25:547
Gandhi KS, Williams MC (1971) J Polym Sci C 35:211
Onogi S, Masuda T, Miyanaga N, Kimure Y (1967) J Polym Sci A-2 5:899
Huggins ML (1942) J Amer Chem Soc 64:2716
Inagaki H, Suzuki H, Fujii M, Matsuo T (1966) J Phys Chem 70:1718
Onogi S, Kobayashi T, Kojima Y, Taniguchi Y (1963) J Appl Polym Sci 7:847
Author information
Authors and Affiliations
Additional information
Dedicated to Prof. Dr. J. Schurz on the occasion of his 60th birthday.
Excerpt from the dissertation of Reinhard Kniewske: „Bedeutung der molekularen Parameter von Polymeren auf die viskoelastischen Eigenschaften in wäßrigen und nichtwäßrigen Medien“, Technische Universität Braunschweig 1983.
Rights and permissions
About this article
Cite this article
Kulicke, W.M., Kniewske, R. The shear viscosity dependence on concentration, molecular weight, and shear rate of polystyrene solutions. Rheol Acta 23, 75–83 (1984). https://doi.org/10.1007/BF01333878
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01333878