Abstract
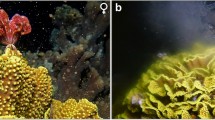
Characteristics of the sexual reproduction and larval settlement of the haplosclerid spongeChalinula sp., which inhabits the shallow waters (1 to 6 m) of Eilat, Red Sea, were investigated from September 1985 through to November 1987. This species was found to be a simultaneous hermaphroditic brooder, hence gonochorism is not the rule in the order Haplosclerida. Brooding always takes place in special brooding chambers. While the oocytes in the brooding chambers are among the largest known in sponges (355±37µm), the spermatic cysts distributed in the choanosome are among the smallest known for this phylum (average 26±7µm).Chalinula sp. breeds throughout the year and in experiments most larvae (74%) settled within 1 to 8 h post-release, generally within 4.5 h. Metamorphosis from larval shape to a sessile sponge lasts 1 to 6 h. Thus, larvae had a short swimming period, settled fast, and metamorphosed rapidly (within 1 to 6 h). The large size of the larvae may contribute to their ability to rapidly reorganize their body shape into that of a sessile sponge. In addition, the existence of already differentiated choanocyte chambers in the larvae, facilitates fast construction of the water filtration system in the newly settled sponges. The reproductive and larval characteristics ofChalinula sp. enable the larvae to settle on any vacant space in the reef, which may explain its abundance in the Red Sea.
Similar content being viewed by others
Literature cited
Babcock, R. C., Bull, G. D., Harrison, P. L., Heyward, A. J., Oliver, J. K., Wallace, C. C., Willis B. L. (1986). Synchronous spawning of 105 scleractinian coral species on the Great Barrier Reef. Mar. Biol. 90: 379–394
Benayahu, Y. (1989). Reproductive cycle and developmental processes during embryogenesis ofClavularia hamra (Cnidaria: Octocorallia). Acta zool., Stock. 70: 29–36
Benayahu, Y., Loya, Y. (1977). Seasonal occurrence of benthic-algae communities and grazing regulation by sea urchins at the coral reefs of Eilat, Red Sea. Proc. 3rd int. Symp. coral Reef 1: 383–389 [Taylor, D. L. (ed.) School of Marine and Atmospheric Sciences, University of Miami]
Benayahu, Y., Loya, Y. (1983). Surface brooding in the Red Sea soft coralParaerythropodium fulvum fulvum (Forskal, 1775). Biol. Bull. mar. biol. Lab., Woods Hole 163: 353–369
Benayahu, Y., Loya, Y. (1984). Life history studies on the Red Sea soft coralXenia macrospiculata Gohar, 1940, II. Planulae shedding and post larval development. Biol. Bull. mar. biol. Lab., Woods Hole 166: 44–53
Benayahu, Y., Loya, Y. (1986). Sexual reproduction of a soft coral: synchronous and brief annual spawning ofSarcophyton glaucum (Quoy & Gaimard, 1833). Biol. Bull. mar. biol. Lab., Woods Hole 170: 32–42
Bergquist, P. R. (1978). Sponges. University of California Press, Los Angeles
Bergquist, P. R., Sinclair, M. E., Green, C. R., Silyn-Roberts, H. (1979). Comparative morphology and behaviour of larvae of Demospongiae. In: Levi, C., Boury-Esnault, N. (eds.) Biologie des spongiaires. C.N.R.S., Colloq. int., Paris 291: 103–111
Boury-Esnault, N., Lopes, M. T. (1985). Les demosponges littorales de l'Archipel des Acores. Ann. Inst. oceanogr., Paris 61: 149–225
Dinesen, Z. D. (1985). Aspects of the life history of the stolon-bearing species ofEfflatounaria (Octocorallia: Xeniidae). Proc. 5th int. coral Reef Congr. 6: 89–94 [Cabrié, C. et al. (eds.) Antenne Museum — EPME, Moorea, French Polynesia]
Elvin, D. W. (1976). Seasonal growth and reproduction of an intertidal sponge,Haliclona permolis (Bowerbank). Biol. Bull. mar. biol. Lab., Woods Hole 151: 108–125
Fadlallah, Y. H. (1983). Sexual reproduction, development and larval biology in scleractinian corals. Coral Reefs 2: 129–150
Fell, P. E. (1976). The reproduction ofHaliclona loosanoffi and its apparent relationship to water temperature. Biol. Bull. mar. biol. Lab., Woods Hole 150: 200–210
Fell, P. E. (1983). Porifera. In: Adiyodi K. G., Adiyodi R. G. (eds.) Reproductive biology of invertebrates. Vol. I, Oogenesis, oviposition, and oosorption. Wiley & Sons, New York, p 1–29
Harrison, P. L., Babcock, R. C., Bull, G. D., Oliver, J. K., Wallace, C. C., Willis, B. L. (1984). Mass spawning in tropical reef corals. Science, N.Y. 233: 1186–1189
Hoppe, W. F. (1988). Reproductive patterns in three species of large coral reef sponges. Coral Reefs 7: 45–50
Hoppe, W. F., Reichert, M. J. M. (1987). Predictable annual mass release of gametes by the coral reef spongeNeofibularia nolitangere (Porifera: Demospongiae). Mar. Biol. 94: 277–285
Ilan, I., Loya, Y. (1990). Reproduction and settlement of the coral reef spongeNiphates sp. (Red Sea). Proc. 6th int. Symp. coral Reef Townsville (in press)
Liaci, L. S., Sciscioli, M., Matarrese, A. (1973). Raffronto tra il comportamento sessuale di alcune ceractinomorpha. Riv. Biol. 66: 135–153
Reiswig, H. M. (1973). Population dynamics of three Jamaican Demospongiae. Bull. mar. Sci. 23: 191–226
Reiswig, H. M. (1983). Porifera. In: Adiyodi, K. G., Adiyodi, R. G. (eds.), Reproductive biology of invertebrates. Vol. II, Spermatogenesis and sperm function. Wiley & Sons, New York, p. 1–21
Shlesinger, Y., Loya, Y. (1985). Coral community reproductive patterns: Red Sea versus the Great Barrier Reef. Science, N.Y. 228: 1333–1335
Soest, R. M. W. van (1980). Marine sponges from Curacao and other Caribbean localities. Part II. Haplosclerida. Stud. Fauna Curacao 62: 1–174
Szmant, A. M. (1986). Reproductive ecology of Caribbean reef corals. Coral Reefs 5: 43–54
Tuzet, O. (1932). Recherches sur l'histologie des eponges. Archs Zool. exp. gen. 74: 169–192
Wapstra, M., Soest, R. M. W. van (1987). Sexual reproduction, larval morphology and behaviour in Demosponges from the South — West of the Netherlands. In: Vacelet, J., Boury-Esnault, N. (eds.) Taxonomy of Porifera. NATO ASI Ser. (G: Ecol. Sciences) 13: 281–307
Weerdt, W. H. de, (1986). A systematic revision of the North-Eastern Atlantic shallow-water Haplosclerida (Porifera, Demospongiae), Part II: Chalinidae. Beaufortia 36: 81–165
Wilkinson, C. R. (1987). Interocean differences in size and nutrition of coral reef sponge populations. Science, N.Y. 236: 1654–1657
Yamazato, K. M., Sato, M., Yamashiro, H. (1981). Reproductive biology of an alcyonacean coralLobophytum crassum Marenzeller. Proc. 4th int. Symp. coral Reef 2: 671–678 [Gomes, E. D. et al. (eds.) Marine Sciences Center, University of the Philippines, Quezan City]
Author information
Authors and Affiliations
Additional information
Communicated by O. Kinne, Oldendorf/Luhe
Rights and permissions
About this article
Cite this article
Ilan, M., Loya, Y. Sexual reproduction and settlement of the coral reef spongeChalinula sp. from the Red Sea. Mar. Biol. 105, 25–31 (1990). https://doi.org/10.1007/BF01344267
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01344267