Abstract
Enthalpies and entropies of sublimation for N-acetylglycine amide (NAGA), N-acetyl-L-alanine amide (L-NAAA), and N-acetyl-D-leucine amide (D-NALA) were determined from the dependence of their vapour pressures on temperature, as measured by the torsion-effusion method.
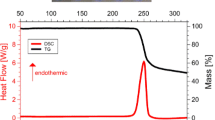
Enthalpies and temperatures of fusion were measured by differential scanning calorimetry (DSC) and entropies of fusion were derived. No solid-to-solid transitions were detected from r.t. to fusion. Enthalpies of sublimation and fusion were combined to evaluate enthalpies of vaporization of the melts.
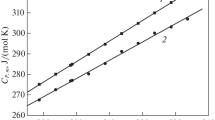
The experimental results decreased inversely to the molecular mass. An interpretation of this trend in terms of the crystalline structure of these compounds is proposed.
Zusammenfassung
Auf Grund der Abhängigkeit des Dampfdruckes von der Temperatur, gemessen mit Hilfe eines Torsions-Effusions-Verfahrens, konnten die Sublimierungsenthalpien und -entropien von N-Azetylglycinamid (NAGA), N-Azetyl-L-alaninamid (L-NAAA) und N-Azetyl-D-leucinamid (D-NALA) bestimmt werden. Schmelztemperatur und Schmelzwärme wurden mit einem Differential Scanning Kalorimeter gemessen und die Schmelzentropie errechnet. Es konnten keine fest- fest Phasenübergänge festgestellt werden. Zur Abschätzung der Verdampfungsenthalpie der Schmelze wurde eine Kombination von Schmelz- und Sublimierungsenthalpie verwendet. Die experimentellen Werte sinken umgekehrt zur molaren Masse. Zur Erklärung dieser Tendenz wird ein auf der Kristallstruktur beruhendes Modell empfohlen.
Резюме
Исходя из измеренной температурной зависимости давлени я паров амидов N-ацетил глицина, N-ацетил-Ь-аланина и N-ац етил-D-лейцина, найдены энтальпии и энтропии из сублимаций. С помощью дифференциальной ск анирующей калориметрии были оп ределены энтальпии и температ уры плавления, а также установлены энтропи и плавления. При этом н е было обнаружено твер дотельных переходов. Энтальпии сублимации и плавлен ия были объединены с целью оп ределения энтальпий испарения расплавов. Экспериме нтальные величины уменьшалис ь обратно пропорцион ально молекулярному весу. И сходя из кристаллической стр уктуры этих соединен ий, предложена интерпре тация этого явления.
Similar content being viewed by others
References
J. Konicek and I. Wadsö, Acta Chem. Scand., 25 (1971) 1541.
G. Della Gatta, L. Stradella and P. Venturello, J. Solution Chem., 10 (1981) 209.
C. H. Spink and I. Wadsö, J. Chem. Thermodynamics, 7 (1975) 561; C. Jolicoeur, B. Riedl, D. Desrochers, L. L. Lemelin, R. Zamojska and O. Enea, J. Solution Chem., 15 (1986) 109.
R. Sköld, J. Suurkuusk and I. Wadsö, J. Chem. Thermodynamics, 8 (1976) 1075.
G. Della Gatta, G. Barone and V. Elia, J. Solution Chem., 15 (1986) 157.
G. M. Blackburn, T. H. Lilley and E. Walmsley, J.C.S. Faraday I, 76 (1980) 915.
R. Puliti, C. A. Mattia, G. Giancola and G. Barone, Acta Cryst., to be published.
R. D. Freeman, The Characterization of High Temperature Vapors, Chapt. 7 (J. L. Margrave, ed.), Wiley, New York, 1967.
V. Piacente and G. De Maria, Ric. Sci. (Rome), 39 (1969) 549.
V. Piacente, P. Scardala, D. Ferro and R. Gigli, J. Chem. Eng. Data, 30 (1985) 372.
R. D. Freeman and J. A. W. Searcy, J. Chem. Phys., 22 (1954) 762.
M. Colomina, P. Jimenez and C. Turrion, J. Chem. Thermodynamics, 14 (1982) 779.
G. Della Gatta and D. Ferro, Thermochim. Acta, 122 (1987) 143.
J. D. Cox and J. Pilcher, Thermochemistry of Organic and Organometallic Compounds, Academic Press, London 1970, p. 125; D. Ferro, G. Barone, G. Della Gatta and V. Piacente, J. Chem. Thermodynamics, 19 (1987) 915.
M. Davies and G. H. Thomas, Trans. Faraday Soc., 55 (1959) 185.
M. Davies and B. Kybett, Trans Faraday Soc., 61 (1965) 1608.
M. Davies and V. E. Malpass, J. Chem. Soc., (1961) 1048.
D. P. Baccanari, J. A. Novinski, Yen-Chi Pan, M. M. Yevitz and H. A. Swain jr., Trans. Faraday Soc., 64 (1968) 1201.
M. Davies, A. H. Jones and G. H. Thomas, Trans. Faraday Soc., 55 (1959) 1100.
R. Puliti, C. A. Mattia and G. Barone, Acta Cryst., to be published.
P. Walden, Z. Elektrochem., 14 (1908) 713.
Author information
Authors and Affiliations
Additional information
Part of the calorimetric measurements was performed by G. Garino while preparing his thesis at the University of Turin. The peptido-amides were synthetised by Dr. G. Giancola during a post-graduate period at the University of Naples. Financial support from the Ministry of Public Education through the Universities of Naples, rome and Turin is gratefully acknowledged.
Rights and permissions
About this article
Cite this article
Ferro, D., Della Gatta, G. & Barone, G. Enthalpies of sublimation and fusion for N-acetyl substituted glycine, L-alanine, and D-leucine amides. Journal of Thermal Analysis 34, 835–841 (1988). https://doi.org/10.1007/BF02331785
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02331785