Summary
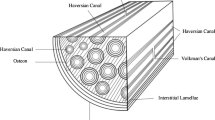
Cortical bone can be modeled as a complex hierarchical composite interrelating both structure and material properties on four levels of structural organization: molecular, ultrastructural, microscopic, and macroscopic. In young animals, the microstructural systems are long parallel lamellar units, plexiform bone, which in older or more mature animals converts by internal remodeling into multiple concentric lamellar units, secondary osteons, forming haversian bone. Ultrasonic wave propagation measurements performed on both plexiform and haversian bone clearly show a definitive relationship with microstructure; haversian bone can be described as a transversely symmetric material whereas plexiform bone appears to be orthotropic in nature. The anisotropy of the elastic constants are found to reflect the tissue symmetry; moreover, plexiform bone is stiffer and more rigid in all directions than is haversian bone. Similar experiments were performed on osteoporotic and osteopetrotic bone. While the results for osteoporotic bone are understandable in terms of the increased porosity, the results for the osteopetrotic bone are anomalous with respect to its density. Since Wolff, the remodeling of bone has been interpreted as a way of altering the mechanical properties to suit some need. For haversian remodeling from plexiform bone, the argument that adaptation occurs to optimize properties requires additional clarification since haversian bone appears to have inferior mechanical properties to plexiform bone.
Similar content being viewed by others
References
Currey JD (1964) Three analogies to explain the mechanical properties of bone. Biorrheol 2:1–10
Currey JD (1969) The relationship between the stiffness and the mineral content of bone. J Biomech 2:477–480
Katz JL (1971) Hard tissue as a composite material. I. Bounds on the elastic behavior. J Biomech 4:455–473
Katz JL (1980a) The structure and biomechanics of bone. In: Vincent JFV, Currey JD (eds) Mechanical properties of biological materials. Cambridge University Press, Cambridge, pp 137–168
Katz JL (1980b) Anistropy of Young's modulus of bone. Nature 283:106–107
Katz JL (1981) Composite material models for cortical bone. In: Cowin SC (ed) Mechanical properties of bone. ASME, New York, AMD 45:171–184
Katz JL, Ukraincik K (1971) On the anisotropic properties of hydroxyapatite. J Biomech 4:221–227
Yoon HS, Katz JL (1976a) Ultrasonic wave propagation in human cortical bone. I. Theoretical considerations for hexagonal symmetry. J Biomech 9:407–412
Yoon HS, Katz JL (1976b) Ultrasonic wave propagation in human cortical bone. II. Measurements of elastic properties and microhardness. J Biomech 9:459–464
Lipson SF, Katz JL (in press) The relationship between elastic properties and microstructure of bovine cortical bone. J Biomech
Morgan B (1973) Osteomalacia, renal osteodystrophy and osteoporosis. Thomas CC (ed) Springfield Illinois, pp 241–338
Jaffe HL (1972) Metabolic degenerative and inflammatory diseases of bones and joints. Lea & Febiger (eds) Philadelphia, pp 178–192
Shapiro F, Glimcher MJ, Holtrop ME, Tashjian AH, Brickley-Parsons D, Kenzora JE (1980) Human osteopetrosis: a histological ultrastructural and biochemical study. J Bone Joint Surg 62-A:384–399
Abendschein W, Hyatt GW (1970) Ultrasonics and selected physical properties of bone. Clin Orthop Rel Res 69:294–301
Johnson LC (1964a) Morphologic analysis in pathology. In: Frost HM (ed) Bone biodynamics. Little, Brown Co, Boston, pp 543–654
Lanyon LE, Magee PT, Baggott DG (1979) The relationship of functional stress and strain to the processes of bone remodeling. An experimental study on the sheep radius. J Biomech 12:593–600
Carter DR, Smith DJ, Spengler DM, Daly CH, Frankel VH (1980) Measurement and analysis of in vivo bone strains on the canine radius and ulna. J Biomech 13:27–38
Currey JD (1975) The effects of strain rate reconstruction and mineral content on some mechanical properties of bovine bone. J Biomech 8:81–86
Wright TM, Hayes WC (1976) Tensile testing of bone over a wide range of strain rates: effects of strain rate microstructure and density. Med Biol Eng 14:671–680
Author information
Authors and Affiliations
Additional information
This research was supported in part by NIDR Grant #DE07054 and NIHR Grant #G00820028
Rights and permissions
About this article
Cite this article
Lawrence Katz, J., Yoon, H.S., Lipson, S. et al. The effects of remodeling on the elastic properties of bone. Calcif Tissue Int 36 (Suppl 1), S31–S36 (1984). https://doi.org/10.1007/BF02406131
Issue Date:
DOI: https://doi.org/10.1007/BF02406131