Abstract
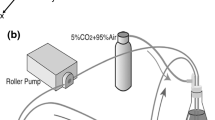
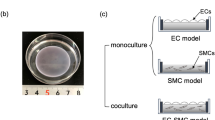
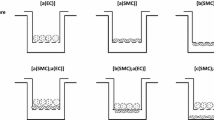
Flow and the associated shear stress have been shown to play an active role in the regulation of the structure and function of endothelial cells (EC)in vitro. Although cultured EC subjected to flow exhibit an elongated morphology and a decreased cell growth rate rather like those observedin vivo, there are differences in morphology and growth rate, as well as other characteristics, betweenin vitro andin vivo EC. This suggests that flow is only one of the many factors affecting EC differentiationin vivo. In this study, a co-culture model system was designed, which includes smooth muscle cells (SMC), a matrix of collagen type I, and a confluent monolayer of EC, and this simplified model of the arterial wall was subjected to a steady, laminar shear stress of 10 and 30 dyn/cm2. Under non-flow conditions, EC exhibited an elongated shape, but with a random orientation. In response to flow, there was an alignment with the direction of flow. This alignment occurred more rapidly at 30 dyn/cm2 than at 10 dyn/cm2. The collagen matrix was found to be primordial in the maintenance of a quiescent endothelium, even in the absence of SMC and flow, suggesting the importance of an organized extracellular matrix (ECM) in the differentiation of cellsin vivo.
Similar content being viewed by others
References
Acevedo, A. D., S. S. Bowser, M. E. Gerritsen, and R. Bizios. Morphological and proliferative responses of endothelial cells to hydrostatic pressure: role of FGF.J. Cell Physiol. 157:603–614, 1993.
Antonelli-Orlidge, A., K. B. Saunders, S. Smith, and P. A. D'Amore. An activated form of transforming growth factor β is produced by cocultures of endothelial cells and pericytes.Proc. Natl. Acad. Sci. USA 86:4544–4548, 1989.
Allen, T. D., S. L. Schor, and A. M. Schor. An ultrastructural review of collagen gels: a model system for cell-matrix, cell-basement membrane and cell-cell interactions.Scanning Electron Microscopy 1984(1):375–390, 1984.
Buck, R. C.. Contact guidance in the subendothelial space. Repair of rat aortain vitro.Exp. Mol. Pathol. 31:275–283, 1979.
Davies, P. F. Vascular cell interactions with special reference to the pathogenesis of atherosclerosis.Lab. Invest. 55(1):5–24, 1986.
Deck, J. D. Endothelial cell orientation on aortic valve leaflets.Cardiovasc. Res. 20:760–767, 1986.
Diamond, S. L., S. G. Eskin, and L. V. McIntire. Fluid flow stimulates tissue plasminogen activator secretion by cultured human endothelial cells.Science 243:1483–1485, 1989.
Frangos, J. A., L. V. McIntire, S. G. Eskin, and C. L. Ives. Flow effects on prostacyclin production by cultured human endothelial cells.Science 227:1477–1479, 1985.
Freudenberg, N. General properties of endothelial cells. In: Fluid Dynamics as a Localizing Factor for Atherosclerosis, edited by G. Schettler, R. M. Nerem, H. Schmid-Schönbein, H. Mörl, and C. Diehm. Berlin: Springer-Verlag, 1985.
Gospodarowicz, D., and C. R. Ill. The extracellular matrix and the control of proliferation of vascular endothelial cells.J. Clin. Invest. 65:1351–1364, 1980.
Harris, A. K., D. Stopak, and P. Wild. Fibroblast traction as a mechanism for collagen morphogenesis.Nature 290:249–251, 1981.
Herman, I. M., A. M. Brant, V. S. Warty, J. Bonaccorso, E. C. Klein, R. L. Kormos, and H. S. Borovetz. Hemodynamics and the vascular endothelial cytoskeleton.J. Cell Biol. 105:291–302, 1987.
Ingber, D. E., and Folkman, J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesisin vitro: role of extracellular matrix.J. Cell Biol. 109:317–330, 1989.
Ives, C. L., S. G. Eskin, and L. V. McIntire. Mechanical effects on endothelial cell morphology:in vitro assessment.In Vitro Cel. Dev. Biol. 22(9):500–507, 1986.
Klebe, R. L., H. Caldwell, and S. Milam. Cells transmit spatial information by orienting collagen fibers.Matrix 9: 451–458, 1989.
Levesque, M. J. and R. M. Nerem. The elongation and orientation of cultured endothelial cells in response to shear stress.J. Biomech. Eng. 107:341–347, 1985.
Levesque, M. J., D. Liepsch, S. Moravec, and R. M. Nerem. Correlation of endothelial cell shape and wall shear stress in a stenosed dog aorta.Arteriosclerosis 6(2):220–229, 1986.
Levesque, M. J., and R. M. Nerem. The study of rheological effects on vascular endothelial cells in culture.Biorhelogy 26(2):345–357, 1989.
Lesvesque, M. J., R. M. Nerem, and E. A. Sprague. Vascular endothelial cell proliferation in culture and the influence of flow.Biomaterials 11:702–707, 1990.
Macarak, E. J., and P. S. Howard. Adhesion of endothelial cells to extracellular matrix proteins.J. Cell. Physiol. 116: 76–86, 1983.
Mitsumata, M., R. M. Nerem, R. W. Alexander, and B. Berk. Shear stress inhibits endothelial cell proliferation by growth arrest in the G0/G1 phase of the cell cycle.FASEB J. 5(4):A527 (904), 1991.
Nishida, K., D. G. Harrison, J. P. Navas, A. A. Fisher, S. P. Dockery, M. Uematsu, R. M. Nerem, R. W. Alexander, and T. J. Murphy. Molecular cloning and characterization of the constitutive bovine aortic endothelial cell nitric oxide synthase.J. Clin. Invest. 90:2092–2096, 1992.
Ookawa, K., M. Sato, and N. Ohshima. Changes in the microstructure of cultured porcine aortic endothelial cells in the early stage after applying a fluid-imposed shear stress.J. Biomech. 25(11):1321–1328, 1992.
Sato, M., M. J. Levesque, and R. M. Nerem. Micropipette aspiration of cultured bovine aortic endothelial cells exposed to shear stress.Arteriosclerosis 7:276–286, 1987.
Silkworth, J. B., and W. E. Stehbens. The shape of endothelial cells inen face preparations of rabbit blood vessels.Angiology 26:474–487, 1975.
Thoumine, O., P. R. Girard, and R. M. Nerem. Effect of shear stress on the extracellular matrix of cultured bovine aortic endothelial cells. (abstract).J. Cell. Biochem. 17E (Suppl.): 157, 1993.
Tokunaga, O., J. L. Fan, and T. Watanabe. Atherosclerosis and endothelium. Properties of aortic endothelial and smooth muscle cells cultured at various ambient pressures.Acta Pathol. Japan 39:356–362, 1989.
Wechezak, A. R., R. F. Viggers, and L. R. Sauvage. Fibronectin and F-actin redistribution in cultured endothelial cells exposed to shear, stress.Lab. Invest. 53(6):639–647, 1985.
Weinberg, C. B., and E. Bell. Regulation of proliferation of bovine aortic endothelial cells, smooth muscle cells and dermal fibroblasts in collagen lattices.J. Cell. Physiol. 122: 410–414, 1985.
Xu, C. B., P. Falke, and L. Stavenow. Interactions between culture bovine arterial smooth muscle cells and endothelial cells; studies on the release of growth inhibiting and growth stimulating factors.Artery 17(6):297–310, 1990.
Ziegler, T. The effect of a steady, laminar shear stress on the proliferation of vascular endothelial cells. Master's Thesis, Georgia Institute of Technology, Atlanta, U.S.A., June 1990.
Ziegler, T., and R. M. Nerem. Tissue engineering a blood vessel: the regulation of vascular biology by mechanical stresses.J. Biol. Chem. 56(2):204–209, 1994.
Ziegler, T., and R. M. Nerem. Co-culture, of endothelial cells with smooth muscle cells in a matrix of collagen: effect of flow on cell morphology (abstract).J. Cell. Biochem. 18C(Suppl.):282, 1994.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ziegler, T., Alexander, R.W. & Nerem, R.M. An endothelial cell-smooth muscle cell co-culture model for use in the investigation of flow effects on vascular biology. Ann Biomed Eng 23, 216–225 (1995). https://doi.org/10.1007/BF02584424
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02584424