Abstract
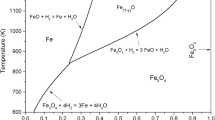
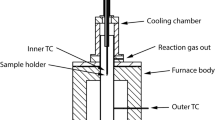
In this mathematical analysis of gaseous reduction of iron oxides, the partial internal reduction of the porous oxide and gas diffusion in the porous iron layer are considered simultaneously in deriving the rate equation. The rate equation, derived by partly analytical and partly numerical solutions, is well substantiated by the experimental results obtained previously. The following parameters, determined previously, are used in the application of the rate equation: i) specific rate constant for the gas reaction on the pore walls of wustite, ii) pore surface area of wustite, iii) effective gas diffusivity in the porous wustite formed during reduction of hematite, and iv) effective gas diffusivity in the porous iron layer. The effective depth of the internal reduction zone at the wustite-iron diffuse interface increases steeply with the progress of reduction beyond about 50 pct O removal. For reduction of 1 to 2 cm diam hematite spheroids in 100 pct H2, the gas composition at the diffuse iron-wustite interface is within 10 to 20 pct of that for the iron-wustite equilibrium; beyond about 50 pct O removal, the rate of reduction is controlled primarily by gas diffusion in the porous iron layer. From the mathematical analysis it is found that the relative depth of internal reduction increases with decreasing particle size and increasing temperature.
Similar content being viewed by others
References
E. T. Turkdogan and J. V. Vinters:Met. Trans., 1971, vol. 2, pp. 3175–88.
E. T. Turkdogan, R. G. Olsson, and J. V. Vinters:Met. Trans., 1971, vol. 2, pp. 3189–96.
E. T. Turkdogan and J. V. Vinters:Met. Trans., 1972, vol. 3, pp. 1561–74.
B. B. L. Seth and H. U. Ross:Trans. Met. Soc. AIME, 1965, vol. 233, pp. 180–85.
R. H. Spitzer, F. S. Manning, and W. O. Philbrook:Trans. Met. Soc. AIME, 1966, vol. 236, pp. 726–42.
R. H. Tien and E. T. Turkdogan:Carbon, 1972, vol. 10, pp. 35–49.
E.W. Thiele: Ind. Eng. Chem., 1939, vol. 31, pp. 916–20.
J. Szekely and J. W. Evans:Met. Trans., 1971, vol. 2, pp. 1691–98 and 1699–1710.
E. T. Turkdogan and J. V. Vinters:Carbon, 1970, vol. 8, pp. 39–53.
E. T. Turkdogan, R. G. Olsson, and J. V. Vinters:Carbon, 1970, vol. 8, pp. 545–64.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tien, R.H., Turkdogan, E.T. Gaseous reduction of iron oxides: Part IV. mathematical analysis of partial internal reduction-diffusion control. Metall Trans 3, 2039–2048 (1972). https://doi.org/10.1007/BF02643212
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02643212