Abstract
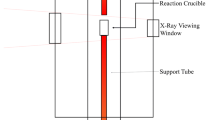
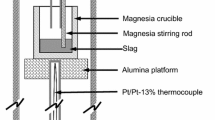
The reduction of chromium oxide from a basic steelmaking slag (45 wt pct CaO, 35 wt pct SiO2, 10 wt pct MgO, 10 wt pct A12O3) by silicon dissolved in liquid iron at steelmaking temperatures was studied to determine the rate-limiting steps. The reduction is described by the reactions: (Cr2O3) + Si = (SiO2) + (CrO) + Cr [1] and 2 (CrO) +Si = (SiO2) + 2 Cr [2] The experiments were carried out under an argon atmosphere in a vertical resistance-heated tube furnace. The slag and metal phases were held in zirconia crucibles. The course of the reactions was followed by periodically sampling the slag phase and analyzing for total chromium, divalent chromium, and iron. Results obtained by varying stirring rate, temperature, and composition defined the rate-limiting mechanism for each reaction. The rate of reduction of trivalent chromium (reaction [1] above) increases with moderate increases in stirring of the slag, and increases markedly with increases in temperature. The effects of changes in composition identified the rate-limiting step for Cr+3 reduction as diffusion of Cr+3 from the bulk slag to the slag-metal interface. The rate of reduction of divalent chromium does not vary with changes in stirring of the slag, but increases in temperature markedly increase the reaction rate. Thus, this reaction is limited by the rate of an interfacial chemical reaction. The reduction of divalent chromium is linearly dependent on concentration of divalent chromium, but is independent of silicon content of the metal phase.
Similar content being viewed by others
References
C. W. McCoy and W. O. Philbrook:The Physical Chemistry of Sleelmaking, pp. 93–98, John WUey and Sons, New York, 1958.
M. G. Frohberg, D. Papamantellos, and E. Hanert:Arch. Eisenhuettenw., 1969, vol. 40, no. l, pp. 15–17.
W. Oelsen, H. Keller, and H. G. Schubert:Arch. Eisenhuettenw., 1970, vol. 41, no. 4, p. 391.
M. Frohberg, K. Richter:Arch. Eisenhuettenw., 1968, vol. 39. no. 11, pp. 799–802.
C. Wagner:The Physical Chemistry of Steelmaking, pp. 237–51, John Wiley and Sons, New York, 1958.
J. W. Robison, Jr.: Ph.D. Dissertation, Rackham Graduate School, The Uni- versity of Michigan, 1973.
F. P. Calderon, N. Sano, and Y. Matsushita:Met. Trans., 1971, vol. 2, pp. 3325–32.
Y. Kawai and K. Mori:J. Iron Steel Inst., Japan 1972, vol. 58, no. 7, pp. 932–53.
M. Paschke and A. Hauptmann:Arch. Eisenhuettenw., 1935, vol. 9, p. 305.
D. W. Morgan and J. A. Kitchener:Trans. Faraday Soc., 1954, vol. 50, p. 51.
E. Grace and G. Derge:Trans. TMS-AIME, 1958, vol. 212, pp. 33–37.
T. Saito, Y. Kawai, K. Maruya, and M. Maki:Physical Chemistry of Process Metallurgy, vol. 1, G. R. St. Pierre, ed., Interscience Publishers, New York, 1961, pp. 523–33.
L. Yang, M. T. Simad, and G. Derge:Trans. AIME, 1956, vol. 206, pp. 1577- 80.
A. C. Riddiford:J. Phys. Chem., 1956, vol. 56, p. 745.
P. J. Koros and T. B. King: Trans. TMS-AIME, 1962. vol. 224. pp. 299–306.
H. Towers and J. Chipman:Trans. AIME, 1957, vol. 209, pp. 769–73.
H. Towers, M. Paris, and J. Chipman:Trans. AIME, 1953, vol. 197, pp. 1455–58.
A. E. Martin and G. Derge:Trans. AME, 1943, vol. 154, p. 104.
N. McCallum and L. R. Barrett:Trans. Brit. Ceram. Soc., 1952, vol. 51, p. 523.
T. Saito and K. Maruya:Sci. Repts. Res. Inst. Tohoku-Univ., 1958, vol. 10, p. 259.
R. F. Johnson:Metals J., Univ. Strathclyde, Glasgow, Scotland, June 1970, no. 20, pp. 33–37
J. Henderson, L. Yang, and G. Derge:Trans. TMS-AIME, 1961, vol. 221, pp. 56–60.
T. Saito and Y. Kawai:Sci. Rept. Res. Inst. Tohoku Univ., 1953, vol. 45, pp. 460–68.
J. I. Mushikin and O. A. Yesin:Doklad. Akad. Nauk. SSSR, 1961 vol. 137, no. 2, p. 388.
J. S. Machin and T. B. Yee:J. Amer. Cer. Soc., 1948, vol. 31, pp. 200–204.
P. Kozakevitch:Physical Chemistry of Process Metallurgy, 1961, Interscience Publishers, New York.
J. S. Machin and D. L. Hanna:J. Amer. Cer. Soc., 1945, vol. 28, pp. 310–16.
J. S. Machin, T. B. Yee, and D. L. Hanna:J. Amer. Cer. Soc., 1952, vol. 35, pp. 322–25.
E. T. Turkdogan and P. M. Bills:Amer. Cer. Soc. Bull., 1960, vol. 39, pp. 682–87.
J. O'M. Bockris and D. C. Lowe:Proc. Royal Soc., 1954, vol. A226, pp. 423–35.
P. Kozakevitch: Rev. Met., 1954, vol. 51 pp. 569–87.
P. Kozakevitch:Rev. Met., 1950, vol. 47, pp. 201–210.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Robison, J.W., Pehlke, R.D. Kinetics of chromium oxide reduction from a basic steelmaking slag by silicon dissolved in liquid iron. Metall Trans 5, 1041–1051 (1974). https://doi.org/10.1007/BF02644316
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02644316