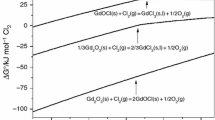
Abstract
Kinetics of chlorination of MoO3 with Cl2-air, Cl2-N2, and Cl2-CO-N2 gas mixtures have been studied by nonisothermal and isothermal thermogravimetric measurements, between ambient temperature and 900 °C. Between 500 °C and 700 °C, the chlorination reaction of MoO3 with Cl2-N2 gas mixture has an apparent activation energy of about 165 kJ/mole, reflecting that a chemical reaction is the rate-controlling step. The reaction order with respect to Cl2 partial pressure is about 0.75. The apparent activation energy for carbochlorination with Cl2-CO-N2 gas mixture is about 83 kJ/mole, between 400 °C and 650 °C. The carbochlorination of MoO3 was controlled by the chemical reaction, probably affected by the pore diffusion regime. The maximum reaction rate is obtained by using a Cl2-CO-N2 gas mixture, having a Cl2/CO volume ratio equal to about 1. The total apparent reaction order with respect to Cl2 + CO in Cl2-CO-N2 gas mixture is about 1.5 for a Cl2/CO ratio equal to 1.
Similar content being viewed by others
References
J.W. Blossom:Molybdenum, U.S. Bureau of Mines, U.S. Government Printing Office, Washington, DC, 1992.
I. Gaballah and M. Djona:Metall. Mater. Trans. B, 1994, vol. 25B, pp. 481–90.
I. Gaballah, M. Djona, and N. Kanari: Final Report of European Community Contracts No. MAIR-0014-C (A), INPL, CRVM, Nancy, France, Feb. 1990.
I. Gaballah and M. Djona: Final Report of European Community Contracts No. MA2R CD 910013, INPL, LEM, Nancy, France, June 1993.
M. Djona: Ph.D. Thesis, INPL, Nancy, France, Jan. 1994.
J.L. Bernard, M. Camelot, and J.L. Taverdet:C.R. Hebd. Seances Acad. Sci., Ser. C, 1974, vol. 279 (26), pp. 1125–28.
M. Camelot, D. Dothée, and F. Billet:Rev. Chim. Miner., 1975, vol. 12 (4), pp. 357–73.
A. Guethert, R. Muenze, and B. Eichler:J. Radioanal. Chem., 1981, vol. 62 (1–2), pp. 91–101.
A.N. Zelikman and L.S. Garba:Sb., Mosk. Inst. Stali Splavov, 1968, No. 45, pp. 70–80.
B.M. Tarakanov, A.N. Zelikman, and V.l. Evdokimov:Zh. Neorg. Khim., 1968, vol. 13 (3), pp. 871–74.
Teofil Mikulski and Bogustawa Jezowska-Trzebiatowska:Przem. Chem., 1970, vol. 49 (10), pp. 590–94.
I.A. Glukhov and S.S. Eliseev:Zh. Neorg. Khim., 1967, vol. 12 (12), pp. 3253–56.
I.A. Glukhov and L.M. Shalukhina:Dokl. Akad. Nauk Tadzh. SSR, 1967, vol. 10(3), pp. 37–40.
M. Del Carmen Ruiz, J.B. Rivalora, and O.D. Quiroga:Lat. Am. Appl. Res., 1990, vol. 20 (2), pp. 107–11.
M.F. Kanunnikov:Zh. Prikl. Khim. (Leningrad), 1986, vol. 59 (8), pp. 1693–97.
S.S. Eliseev, E.E. Vozhdaeva, L.E. Malysheva, and N.V. Gaidaenko:Izv. Akad. Nauk Tadzh. SSR, Otd. Fiz.-Mat. Geol.-Khim. Nauk, 1981, vol. (4), pp. 87–90.
A. Roine:Outokumpu HSC Chemistry for Windows, version 2.0, Outokumpu Research, Pori, Finland, June 1994.
J. Szekely, J.W. Evans, and H.Y. Sohn:Gas-Solid Reactions, Academic Press, New York, NY, 1976, pp. 115–7 and 232-39.
P. Pascal:Nouveau Traité de Chimie Minérale, Tome XIV, Masson et Cie Editeurs, Paris, 1959, pp. 656–58.
G. Liljestrand:Reactivity of Solids, Proc. 8th Int. Symp. on the Reactivity of Solids, Goteborg, June 14-19, 1976, J. Wood,O. Lindqvist, C. Helgesson, and N.-G. Vannerberg, eds., Plenum Press, New York, NY, 1977, p. 385.
Author information
Authors and Affiliations
Additional information
Laboratoire Environnement et Minéralurgie, associated with the Centre National de la Recherche Scientifique, Mineral Processing and Environmental Engineering team.
Rights and permissions
About this article
Cite this article
Djona, M., Allain, E. & Gaballah, I. Kinetics of chlorination and carbochlorination of molybdenum trioxide. Metall Mater Trans B 26, 703–710 (1995). https://doi.org/10.1007/BF02651716
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02651716