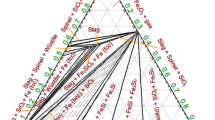
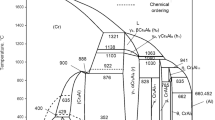
Abstract
Available thermodynamic and phase diagram data have been critically assessed for all phases in the CrO-Cr2O3, CrO-Cr2O2-Al2O3, and CrO-Cr2O2-CaO systems from 298 K to above the liquidus temperatures and for oxygen partial pressures ranging from equilibrium with metallic Cr to equilibrium with air in the case of the first two systems and toP O 2 = 10−3 atm for the CrO-Cr2O3-CaO system. All reliable data have been simultaneously optimized to obtain one set of model equations for the Gibbs energy of the liquid slag and all solid phases as functions of composition and temperature. The modified quasichemical model was used for the slag. The models permit phase equilibria to be calculated for regions of composition, temperature, and oxygen potential where data are not available.
Similar content being viewed by others
Cited References
G. Eriksson, P. Wu, and A.D. Pelton,Calphad, 17, 189–206 (1993).
P. Wu, G. Eriksson, A.D. Pelton, and M. Blander,J. Iron Steel Inst. Jpn., 33, 25–34 (1993).
G. Eriksson and A.D. Pelton,Metall. Trans. B, 24, 795–805 (1993).
G. Eriksson and A.D. Pelton,Metall. Trans. B, 24, 807–816 (1993).
P. Wu, G. Eriksson, and A.D. Pelton,J. Am. Ceram. Soc., 76, 2059–2064 (1993).
P. Wu, G. Eriksson, and A.D. Pelton,J. Am. Ceram. Soc., 76, 2065–2075 (1993).
G. Eriksson, P. Wu, M. Blander, and A.D. Pelton,Can. Metall. Q., 33, 13–22 (1994).
G. Eriksson, A.D. Pelton, E. Woermann, and A. Ender,Ber. Bun-senges. Phys. Chem., in press.
S. Degterov and A.D. Pelton,J. Phase Equilibria, 77(6), 488–494 (1996).
S. Degterov and A.D. Pelton,Metall. Trans., in press.
A.D. Pelton and M. Blander,Proc. Second Int. Symp. Metall. Slag and Fluxes, TMS-AIME, Warrendale, PA, 281–291 (1984).
M. Blander and A.D. Pelton,Proc. Second Int. Symp. Metall. Slags and Fluxes, TMS-AIME, Warrendale, PA, 294–304 (1984).
A.D. Pelton and M. Blander,Metall. Trans. B, 17, 805–815 (1986).
M. Blander and A.D. Pelton,Geochim. Cosmochim. Acta, 51, 85–95 (1987).
A.D. Pelton and M. Biander,Calphad, 12, 97–108 (1988).
M.L. Kapoor and M.G. Frohberg,Symp. Chem. Metall. Iron and Steel, Sheffield, England, The Institute of Metals, London (1971).
H. Gaye and J. Weifringer,Proc. Second Int. Symp. Metall. Slags and Fluxes, TMS-AIME, Warrendale, PA, 357–376 (1984).
G.W. Toop and C.S. Samis,TMS-AIME, 224, 878 (1962).
P. Wu, Ph.D. Thesis, École Polytechnique, Montréal (1992).
A.D. Pelton, G. Eriksson, and M. Blander,Proc. Third Int. Symp. Metall. Slags and Fluxes, The Institute of Metals, London, 66–69 (1989).
A.D. Pelton and G. Eriksson,Advances in the Fusion of Glass, Proc. First Int. Conf. on Advances in the Fusion of Glass, American Ceramic Society, Westerville, OH, 27.1–29.11 (1988).
A.D. Pelton, W.T. Thompson, C.W. Bale, and G. Eriksson,Advances in Phase Transitions, J.D. Embury and G.R. Purdy, Ed., Pergamon Press, New York, NY, 52–67 (1988).
M. Blander, A.D. Pelton, and G. Eriksson,Proc. Fourth Int. Symp. Metall. Slags and Fluxes, Iron Steel Institute of Japan, Tokyo, 56–60 (1992).
JANAFThermochemical Tables, 3rded.,J.Phys. Chem. Ref. Data, 14(1985).
Y. Jeannin, C. Mannerskantz, and F.D. Richardson,TMS-AIME, 227, 300 (1963).
D. Tretyakov and H. Schmalzried,Ben Bunsenges. Phys. Chem., 69, 396 (1965).
N.Y. Toker, L.S. Darken, and A. Muan,Metall. Trans. B, 22, 225–232 (1991).
F.N. Mazandarany and R.D. Pehlke,J. Electrochem. Soc., 121, 711–714 (1974).
H. Davies and W.W. Smeltzer,J. Electrochem. Soc., 121, 543–549 (1974).
A. Dinsdale,Calphad, 15, 317–426 (1991).
J.R. Taylor and A.T. Dinsdale,Z. Metallkd., 81, 354–366 (1990).
G. Banik, T. Schmitt, P. Ettmayer, and B. Lux,Z. Metallkd., 71, 644–645 (1980).
Y.I. Ol'shanskii and V.S. Shlepov,Dokl. Akad. Nauk SSSR, 91, 561–564 (1953).
K.K. Kelley, F.S. Boericke, G.E. Moore, E.H. Huffmann, and W.M. Bangert, U.S. Bur. Mines Tech. Paper No. 662 (1994).
T. Matsui and K. Naito,J. Nucl. Mater., 136, 78–82 (1985).
E.N. Bunting,Bur. Std. J. Res., 6, 947–949 (1931).
A. Neuhaus, E. Jumpertz, and P. Brenner,Fortschr. Miner., 40, 60 (1963).
D.M. Roy and A.E. Barks,Nature, 235, 118–119 (1972).
K.T. Jacob,J. Electrochem. Soc., 125, 175–179 (1978).
H.-T.T. Tsai and A. Muan,J. Phase Equilibria, 75, 1412–1415 (1992).
N.D. Chatterjee, H. Leistner, L. Terhart, K. Abraham, and R. Klaska,Am. Mineral, 67, 725–735 (1982).
W. Sitte,Mater. Sci. Monogr., 28A, 451–456 (1985).
T. Sasamoto and T. Sata,Bull. Tokyo last. Technol., 108, 123–139 (1972).
C.O. Hulse, Final Technical Report, Contract No. 0019-74-C-0271 (1975).
A. Muan,High Temp.-High Pmss., 14, 653–660 (1982).
T. Schmitt, G. Banik, and B. Lux,Ber. Deutsche Keram. Ges., 57, 80–83 (1980).
W.F. Ford and W.J. Rees, Appendix by J. White,Trans. Brit. Ceram. Soc., 48, 291–321 (1949).
W.F. Ford and W.J. Rees,Trans. Brit. Ceram. Soc., 47, 207–231 (1948).
W.F. Ford and J. White,Trans. Brit. Ceram. Soc., 48, 417–427 (1949).
V. Danek and T. Licko,Silikaty, 25, 55–60 (1981).
Z. Panek,Silikaty, 25, 169–171 (1981).
A. Kaiser, B. Sommer, and E. Woermann,J. Am. Ceram. Soc., 75, 1463–1471 (1992).
F.P. Glasser and E.F. Osbom,J. Am. Ceram. Soc., 41, 358–367 (1958).
K. Wilhelmi and O. Jonsson,Acta Chem. Scand., 19, 177–184 (1965).
K.T. Adendorif, J.P.R. De Villiers, and GJ. Kruger,J. Am. Ceram. Soc., 75, 1416–1422 (1992).
J.P.R. De Villiers, J. Mathias, and A. Muan,Trans. Inst. Min. Metall., 96 (Sect. C: Mineral Process. Extr. Metall.), C55–62 (1987).
H. von Wartenberg, H.J. Reusch, and E. Saran,Z. Anorg. Allg. Chem., 230, 257–276 (1937).
Ya.I. Ol'shanskii, A.I. Tsvetkov, and V.K. Shlepov,Dokl. Akad. Nauk SSSR, 96, 1007–1009 (1954).
Z. Panek,Silikaty, 20, 1–12 (1976).
A. Muan,Spec. Publ. Geol. Soc. S. Afr., 7, 325–336 (1983).
J.P.R. De Villiers and A. Muan,J. Am. Ceram. Soc., 75, 1333–1341 (1992).
F. Bertaut, P. Blum, and G. Magnano,C. R. Acad. Sci. Paris, 241, 757–759 (1955).
P.M. Hill, H.S. Peiser, and J.R. Rait,Acta Crystallogr., 9, 981–986 (1956).
F.P. Glasser and L.S. Dent,Inorg. Chem., 7, 428–429 (1962).
H.Von Pausch and H.k. Muller-Buschbaum,Z. Anorg. Allg. Chem., 405, 113–118 (1974).
Z. Panek and E. Kanclir,Silikaty, 20, 113–122 (1976).
J..Havlica and Z. Panek,Silikaty, 24, 1–6 (1980).
K.V. Rajagopalan, R. Kalyanaraman, and M. Sundaresan,J. Electrochem. Soc. India, 34, 229–231 (1985).
M.L. Kovba, Yu.Ya. Skolis and S.G. Popov,Zh. Fiz, Khim., 64, 40–44 (1990).
J. Havlica and Z. Panek,Silikaty, 21, 13–18 (1977).
J. Havlica and V. Ambruz,Thermochim. Acta, 93, 337–340 (1985).
M.L. Kovba and Yu.Ya. Skolis,Zh. Fiz. Khim., 61, 330–334 (1987).
G.F. Voronin and S.A. Degterov,Physica C, 176, 387–408 (1991).
S.A. Degterov and G.F. Voronin,Physica C, 178, 213–220 (1991).
I. Barin,Thermochemical Data of Pure Substances, VCH, Weinheim, Germany (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Degterov, S., Pelton, A.D. Critical evaluation and optimization of the thermodynamic properties and phase diagrams of the CrO-Cr2O3, CrO-Cr2O3-Al2O3, and CrO-Cr2O3-CaO systems. JPE 17, 476–487 (1996). https://doi.org/10.1007/BF02665994
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02665994