Abstract
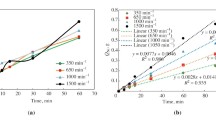
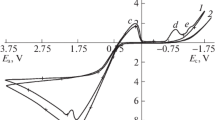
The rotating disk technique was used to study silver dissolution with thiourea as a function of sulfuric acid, ferric sulfate, and thiourea concentrations. The effect of many foreign ions (Mn, Cu, Co, Ca, Na,etc.) and various additives was also examined. The dissolution of silver was zero order with sulfuric acid, first order with ferric sulfate, and second order with thiourea. Among the foreign ions, copper had a dramatically negative effect. The strong oxidants such as hydrogen peroxide and manganese dioxide were also detrimental for silver dissolution. According to the temperature effect studied (5 °C to 35 °C), the activation energy was 22.6 kJ/ mole. Silver does not dissolve with thiourea in the absence of ferric ions. Sulfuric acid does not participate in the dissolution reaction. The most important parameter for silver dissolution is the ferric sulfate/thiourea ratio. In excess ferric sulfate, a solid silver-thiourea complex is formed, which precludes transfer of silver into solution. In excess thiourea, the free thiourea reacts with formed solid silver-thiourea complex, and silver goes into the solution, predominantly as the dimers of AgTU+ 3 complexes. The solid silver-thiourea complex in question was characterized by various spectroscopic, microscopic, and chemical analysis techniques. According to chemical composition, it corresponds to Ag2SO2·3TUH2O compound.
Similar content being viewed by others
References
I. N. Plaksin and M. A. Kozhuchova:Compt. Rend. (Dokl.)Acad. Sci. URSS, Doklady Akademii Nauk SSSR, 1941, vol. 31, pp. 671–74.
C. K. Chen, T. N. Lung, and C. C. Wan:Hydrometallurgy, 1980, vol. 5, pp. 207–12.
A. F. Panchenko, I. A. Kakovskiy, L. A. Shamis, and O. D. Khmel’nitshaya:Izv. Akad. Nauk SSSR, Met., 1975, vol. 6, pp. 32–37.
J. C. Huyhua and I. H. Gundiler:Proc. Int. Symp. on Hydrometallurgical Reactor Design and Kinetics, AIME-TMS, Sept. 1986.
R. G. Schulze:J. Met., June 1984, pp. 62-65.
V. G. Levich:Physicochemical Hydromechanics, Prentice-Hall, Englewood Cliffs, NJ, 1962.
B. Pesic: Final Report to the National Science Foundation, Grant No. MSM-8306337, July 25, 1986, 91 pp.
O. D. Khmel’nitskaya, T. D. Gornostaeva, A. F. Panchenko, and V. V. Lodeishchikov:Russ. J. Inorg. Chem., 1985, vol. 30 (2), pp. 319–20.
T. D. Gornostaeva, O. D. Khmel’nitskaya, A. F. Panchenko, and V. V. Lodeishchikov.Th. Neorg. Khim., 1986, vol. 31, pp. 115–18.
J. Maslowska:Roczniki Chemii, 1968, vol. 42, pp. 1191–98.
R. A. Bailey and T. R. Peterson:Can. J. Chem., 1967, vol. 45, pp. 1135–42.
N. Pustelnik and R. Soloniewicz:Monatsh. Chem., 1975, vol. 106, pp. 673–78.
N. Pustelnik and R. Soloniewicz:Monatsh. Chem., 1978, vol. 109, pp. 33–40.
P. M. Henrichs, J. J. Ackerman, and G. E. Maciel:J. Am. Chem. Soc, 1977, vol. 99 (8), pp. 2544–48.
Author information
Authors and Affiliations
Additional information
Formerly Graduate Student, University of Idaho
Rights and permissions
About this article
Cite this article
Pesic, B., Seal, T. A rotating disk study of silver dissolution with thiourea in the presence of ferric sulfate. Metall Trans B 21, 419–427 (1990). https://doi.org/10.1007/BF02667853
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02667853