Abstract
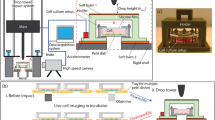
A novelin vitro system was developed to examine the effects of traumatic mechanical loading on individual cells. The cell shearing injury device (CSID) is a parallel disk viscometer that applies fluid shear stress with variable onset rate. The CSID was used in conjunction with microscopy and biochemical techniques to obtain a quantitative expression of the deformation and functional response of neurons to injury. Analytical and numerical approximations of the shear stress at the bottom disk were compared to determine the contribution of secondary flows. A significant portion of the shear stress was directed in ther-direction during start-up, and therefore the full Navier-Stokes equation was necessary to accurately describe the transient shear stress. When shear stress was applied at a high rate (800 dyne cm−2 sec−1) to cultured neurons, a range of cell membrane strains (0.01 to 0.53) was obtained, suggesting inhomogeneity in cellular response. Functionally, cytosolic calcium and extracellular lactate dehydrogenase levels increased in response to high strain rate (>1 sec−1) loading, compared with quasistatic (<1 sec−1) loading. In addition, a subpopulation of the culture subjected to rapid deformation subsequently died. These strain rates are relevant to those shown to occur in traumatic injury, and as such, the CSID is an appropriate model for studying the biomechanics and pathophysiology of neuronal injury.
Similar content being viewed by others
References
Barbee, K. A., E. J. Macarak, and L. E. Thibault. Strain measurements in cultured vascular smooth muscle cells subjected to mechanical deformation.Ann. Biomed. Eng. 22: 14–22, 1994.
Cargill, R. S., and L. E. Thibault. Acute alterations in [Ca2+]i in NG108-15 cells subjected to high strain rate deformation and chemical hypoxia: anin vitro model for neural trauma.J. Neurotrauma 13:395–407, 1996.
Davies, P. F., F. Dewy, S. R. Bussolari, E. J. Gordon, and M. A. Gimbrone. Influence of hemodynamic forces on vascular endothelial function.J. Clin. Invest 73:1121–1129, 1984.
Dewey, C. F. J., and S. R. Bussolari. The dynamic response of vascular endothelial cells to fluid shear stress.J. Biomech. Eng. 103:177–185, 1981.
Ellis, E. F., J. S. McKinney, K. A. Willoughby, S. Liang, and J. T. Polishock. A new model for rapid stretch-induced injury of cells in culture: characterization of the model using astrocytes.J. Neurotrauma 12:325–339, 1995.
Evans, E., and B. Kukan. Passive mechanical behavior of granulocytes based on large deformation and recovery after deformation tests.Blood 64:1028–1035, 1984.
Galbraith, J., and L. E. Thibault. Mechanically induced depolarizations in the squid giant axon.J. Biomech. Eng. 115: 13–22, 1993.
Ganot, G., B. Wong, L. Binstock, and G. Eherenstein. Reversal potentials corresponding to mechanical stimulation and leakage current in Myxicola giant axons.Biochim. Biophys. Acta 649:487–491, 1981.
Gennarelli, T. A.. Mechanisms of brain injury.J. Emerg. Med. 11:5–11, 1993.
Gennarelli, T. A., and L. E. Thibault. Biological models of head injury. In: Central nervous system trauma status report, edited by J. T. Povlishock, Bethesda, MD: NINCDS, NIH, Public Health Services, 1984.
Graham, D. I., J. H. Adams, D. Doyle, I. Ford, T. A. Gennarelli, A. E. Lawrence, W. L. Maxwell, and D. R. McLellan. Quantification of primary and secondary lesions in severe head injury.Acta Neurochir. 57:41–48, 1993.
Guilak, F.. Volume and surface area measurement of viable chondrocytesin situ using geometric modelling of serial confocal sections.J. Microsc. 173:245–256, 1994.
Hyman, W. A.. Shear flow over a protrusion from a plane wall.J. Biomech. 5:45–48, 1972.
Landsman, A. S., D. F. Meaney, R. S. Cargill, E. J. Macarak, and L. E. Thibault. 1995 William J. Stickel Gold Award. High strain rate tissue deformation: a theory on the mechanical etiology of diabetic foot ulcerations.J. Am. Podiatr. Med. Assoc. 85:519–527, 1995.
Leal, G. L. Laminar Flow and Convective Processes: Scaling Principles and Asymptotic Analysis. Boston: Butterworth-Heinemann, 1992.
Levich, V. G. Physicochemical Hydrodynamics, Englewood Cliffs, NJ: Prentice-Hall, Inc., 1962.
Lucas, J. H., and A. Wolf.In vitro studies of multiple impact injury to mammalian CNS neurons: prevention of perikaryal damage and death by ketamine.Brain Res. 543:181–193, 1991.
Margulies, S. S., L. E. Thibault, and T. A. Gennarelli. Physical model simulations of brain injury in the primate.J. Biomech. 23:823–836, 1990.
Nomura, H., C. Ishikawa, T. Komatsuda, J. Ando, and A. Kamiya. A disk-type apparatus for applying fluid shear stress on cultured endothelial cells.Biorheology 25:461–470, 1988.
Orrenius, S., D. J. McConkey, G. Bellomo, and P. Nicotera. Role of Ca2+ in toxic cell killing.Trends Pharmacol. Sci. 10:281–285, 1989.
Pleasure, S. J., C. Page, and V. M. Y. Lee. Pure, postmitotic, polarized human neurons derived from NTera 2 cells provide a system for expressing exogenous proteins in terminally differentiated neurons.J. Neurosci. 12:1802–1815, 1992.
Rand, R. P., and A. C. Burton. Mechanical properties of the red cell membrane.Biophys. J. 4:115–135, 1964.
Saatman, K. E., and L. E. Thibault. Axonal injury studied in a single myelinated nerve fiber model.J. Neurotrauma 11: 125, 1994.
Sato, M., D. P. Theret, L. T. Wheeler, N. Ohshima, and R. M. Nerem. Application of the micropipette technique to the measurement of cultured porcine aortic endothelial cell viscoelastic properties.J. Biomech. Eng. 112:263–268, 1990.
Schanne, F. A. X., A. B. Kane, E. E. Young, and J. L. Farber. Calcium dependence of toxic cell death: a final common pathway.Science 206:700–702, 1979.
Shepard, S. R., J. B. G. Ghajar, R. Giannuzzi, S. Kupferman, and R. J. Hariri. Fluid percussion barotrauma chamber: a newin vitro model for traumatic brain injury.J. Surg. Res. 51:417–424, 1991.
Siesjo, B. K.. Basic mechanisms of traumatic brain damage.Ann. Emerg. Med. 22:959–969, 1993.
Simon, S. I., and G. W. Schmid-Schonbein. Cytoplasmic strains and strain rates in motile polymorphonuclear leukocytes.Biophys. J. 58:319–332, 1990.
Thibault, L. E.. Isolated tissue and cellular biomechanics. In: Accidental injury: biomechanics and prevention, edited by R. P. Nahum and J. W. Melvin. New York: Springer-Verlag & Co., 1993, pp. 512–537.
Trump, B. F., and I. K. Berezesky. The role of cytosolic Ca2+ in cell injury, necrosis and apoptosis.Curr. Opin. Cell Biol. 4:227–232, 1992.
Watson, P. A.. Function follows form: generation of intracullular signals by cell deformation.FASEB J. 5:2013–2019, 1991.
Winston, F. L., E. J. Macarak, S. F. Gorften, and L. E. Thibault. A system to reproduce and quantify the biomechanical environment of the cell.J. Appl. Physiol. 67:397–405, 1989.
Zimmermann, U., G. Pilwat, A. Pequeux, and R. Gilles. Electro-mechanical properties of human erthyrocyte membranes: The pressure-dependence of potassium permeability.J. Mem. Biol. 54:103–113, 1980.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
LaPlaca, M.C., Thibault, L.E. Anin vitro traumatic injury model to examine the response of neurons to a hydrodynamically-induced deformation. Ann Biomed Eng 25, 665–677 (1997). https://doi.org/10.1007/BF02684844
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02684844