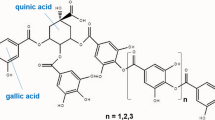
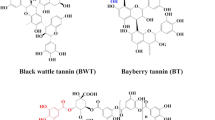
Conclusions
1. The analytical procedure outlined previously is recommended for the evaluation of the interaction of tannins with iron species. Prior to formulation, each tannin must be evaluated to assess its usefulness in formulating anticorrosive primers.
2. The determination of pH and corrosivity of tannin suspensions may help in understanding its interaction with steel. The more acidic and the more corrosive the suspension, the greater the interaction with steel.
3. The optimum pH for the tannate film lies in the range 3.0–5.0. The values are close to the natural pH of the tannins suspensions.
4. The presence of phosphoric acid in the formulations passivates the steel surfaces and favours the formation of a tannate film on clean steel surfaces. Also it assists dissolution of the oxides and further reaction with tannins.
5. Tannins react with clean and rusted steel surfaces creating a layer with a plate-like structure replacing the globular one of iron oxides.
6. Aqueous primers containing tannins inhibit steel corrosion as was revealed by potential corrosion measurements.
Similar content being viewed by others
References
Thomas NL,J. Protect. Coat. & Lin., (12), 63–71, 1989.
Winkel P,Galvanotechnik,83, (9), 3095–97, 1992.
White J,Corrosion Reviews,7, (2 & 3), 235–257, 1987.
Joseph G and Vallejos R,Rev Iber. Corros. y Prot.,XIX, (6), 379–381, 1988.
Kuzniecow JI and Iwanow J,Ochrona Przed Koroz.,33, (8–9), 1990.
Morcillo M, Feliú S, Simancas J, Bastidas JM, Galvan J, Feliú S (Jr) and Almeida EM,Corrosion (NACE),48, (12), 1031–1039, 1992.
González MI and Abreu A,Revistas de Ciencias Químicas,15, 315–317, 1984.
A. J. Seavell,J. Oil Col. Chem. Assoc.,61, (12), 439–462, 1978.
DesLauriers PJ,Mat. Perf.,26, (11), 35–40, 1987.
Alvarez ZE, Callozo I and Valdés D,Rev. Iber. Corros. y Prot.,XVIII, (1), 35–38, 1987.
Nigam AM, Tripathi RP and Dhoot K,Corr. Sci.,30, (8–9), 799–803, 1990.
González MI and Corvo F,Rev. Iber. Corros. y Prot.,XVIII, 39–41, 1987.
Kurkurs O,Produkti atmosfernoi korrozii zheleza i obraska porzhauchine, Ed. Zinatne, Riga, 83–86, 1980.
Gianelli R,Memorias del II Congreso Iberoamericano de Corrosión y Protección, 253–257, Venezuela, 1986.
Morcillo M, Feliú S and Simancas J,Farbe + Lack,95, (10), 726–728, 1989.
Emeric DA and Miller CE,Proc. ADV MAT/91 (NACE, Houston), 212, San Diego, 1991.
Guruviah S and Sundaram M,Proc. Adv. Surf Treatment Metals, 216–20, Bombay, 1987.
Lin Ch, Lin P, Hsiao M, Meldrum DA and Martin FL,Ind. Eng. Chem. Res.,31, (1), 424–430, 1992.
Watanabe T, Kanda S and Oono H,JP 62,260,866 [87,260,866] (Cl. C09D3/58), 1987.
Szego F, Szego FM Patel and Szego K,Hung. Teljes HU 43,100 (Cl C08L33/08) 28SEP1987.
NIPPON OILS & FATS CO.,Jap. Pat. Abs. 92 (21) Gp G, 54. Patent Number 04/110357.
PENZOIL PRODUCTS CO. —United States Patent 5015507: Off. Gaz. (2), 1036, 1991.
Balmforth B,Proc. ‘Corrosion 91’, Cincinnati, Paper 561.
Balmforth B,Chemicals for the Automotive Industry, Drake J. G. (Ed.), p. 38–46, Royal Society of Chemistry, London, 1991.
Bruzzoni WO, Aznar A and Iñiguez Rodríguez A,Rev. Iber. Corros. y Prot.,6 (2), 3–10, 1975.
Morcillo M, Gracia M, Gancedo JR and Feliú S,Memorias del II Congreso Iberoamericano de Corrosión y Protección, 221–228, Venezuela, 1986.
Seavell AJ,J. Oil Color Chem. Assoc,75, (8) 293–306, 1992.
Draper PA,J. Oil Color. Chem. Assoc.,68, (10), 243–250, 1985.
Haslam E,Chemistry of Vegetable tannins, Academic Press Inc. Ltd., London, 1966.
Ross TK and Francis RA,Corr. Sci.,18, 351–361, 1978.
Noller CR,Química de los Compuestos Orgánicos, p. 758–759, López Libreros Editores S. R. L., Buenos Aires, 1968.
Bermejo Martínez F and Prieto Bouza A, Aplicaciones analíticas del AEDT y similares, p. 28, Imprenta del Seminario Conciliar, Santiago de Compostela, 1960.
Gust J and Wawer I,Corrosion (NACE),51, (1), 37–44, 1995.
Brandau AH,Introduction to coatings technology, p. 40, Federation Series for Coatings Technology, USA, 1990.
Uhlig HH,The Corrosion Handbook, p. 910–912, John Wiley & Sons, Inc., N.Y., 1948.
del Amo B, Romagnoli R and Vetere VF,Corrosion Reviews,XIV, (1–2), 121–134, 1996.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vetere, V.F., Romagnoli, R. Chemical and electrochemical assessment of tannins and aqueous primers containing tannins. Surface Coatings International 81, 385–391 (1998). https://doi.org/10.1007/BF02693869
Issue Date:
DOI: https://doi.org/10.1007/BF02693869