Abstract
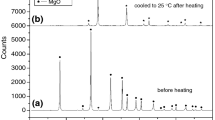
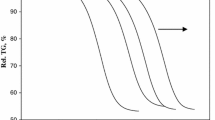
The kinetic parameters of the thermal decomposition of boric acid have been investigated by using TGA data. Suzuki and Coats-Redfern methods have been applied for the kinetic investigation. It was determined that decomposition kinetics of boric acid occurred in two steps and both regions suitably fit a first-order kinetic model. According to Coats-Redfern method, the activation energy and frequency factor were found as 79.85 kJ·mol-1 and 3.82x 104 min-1 for region I and 4.79 kJ·mol-1 and 4.045 x 10-5 min-1 for region II, respectively. The activation energies and frequency factors were found as 4.45 kJ·mol-1 and 4.08 x108 min-1 for the Suzuki method.
Similar content being viewed by others
References
Coats, A. W. and Redfern, J. P.,“Kinetic parameters from thermogravimetric data,”Nature,201, 68 (1964).
Demir, F., Dönmez, B., Okur, H. and Sevim, E., “Calcination kinetic of magnesite from thermogravimetric data,”Trans IChemE,81, 918 (2003).
Ersahan, H., Ekmekyapar, A. and Sevim, F., “Flash calcination of magnesite ore in a free-fall reactor and leaching of magnesia,”Int. J. Miner. Process,42, 121(1994).
KocakuŞak, S., AkÇay, K., Ayok, T., Köroğlu, J., SavaŞÇ, T. and ve Tolun, R.,“AkiŞkan yatakta bor,” Tübitak AraŞtirma Merkezi, Rapor No:KM323 (1998).
Kraschwit, J. I. (Ed.),Kirk-othmer enyclopedia of chemical technology, John Wiley and Sons, New York, 22 (1997).
Marano, R. T. and Shuster, E. R.,Inorganic materials and physical chemistry, R. F. Schuwenber and P. D. Garn (Eds.), Academic Press, New York, 709 (1969).
Okur, H. and Eymir, Ç.,“Dehydration kinetics of ulexite by thermogravimetric data using the coats-redfern and genetic algorithm method,”Ind. Eng. Chem. Res.,42, 3642 (2003).
Olszak-Humienik, M. and Mozejko, J.,“Kinetics of thermal decomposition of dolomite,”Journal of Thermal Analysis and Calorimetry,56, 829 (1999).
Park, J. W., Oh, S. C., Lee, H. P., Kim, H. T. and Yoo, K. O.,“Kinetic analysis of thermal decomposition of polymer using a dynamic model,”Korean J. Chem. Eng.,17, 489 (2000).
PiŞkin, S., PhD Thesis, İstanbul Technical University, Metallurgy Dept. İstanbul, Turkey, 22 (1983).
Suzuki, M., Misic, D. M., Koyama, O. and Kawazoe, K.,“Study of thermal regeneration of spent activated carbons: thermogravimetric measurement of various single component organics loaded on activated carbons,”Chem. Eng. Sci,33, 271 (1978).
ŞÇener, S., özbayoğlu, G. and Demirci, Ş.,“Changes in the structure of ulexite on heating,”Thermochimica Acta,362, 107 (2000).
The Economics of Boron, Roskill Information Service Ltd. (1995).
Yun, Y. and Lee, G.,“Effects of pressure in coal pyrolysis observed by high pressure TGA,”Korean J. Chem. Eng.,16, 798 (1999).
Zachariasen, W. H.,“The crystal structure of cubic metaboric acid,”Acta Crystallogr.,16, 380 (1963).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sevim, F., Demir, F., Bilen, M. et al. Kinetic analysis of thermal decomposition of boric acid from thermogravimetric data. Korean J. Chem. Eng. 23, 736–740 (2006). https://doi.org/10.1007/BF02705920
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02705920