Abstract
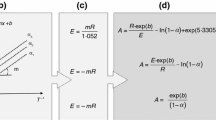
This paper presents a new approach to estimate the Arrhenius parameters as well as the reaction model function of cellulose pyrolysis reaction. Reduced time plot (RTP) was employed to choose a proper form of reaction model function for cellulose pyrolysis reaction. A state-of-the-art thermobalance (TB) that is able to form real isothermal reaction conditions was introduced to construct RTPs from isothermal decomposition kinetic data. The reaction model function of cellulose pyrolysis reaction would be accounted for by Avrami-Erofeev function, n(1-α){-ln(1-a)}1-1/n where n is determined to be 3.69.
Similar content being viewed by others
References
Agrawal, R., “Kinetics of reactions involved in pyrolysis of cellulose I. The three reaction model,”Can. J. Chem. Eng.,66, 403 (1988a).
Agrawal, R., “Kinetic of reactions involved in pyrolysis of cellulose II. The modified Kilzer-Broido model,”Can. J. Chem. Eng.,66, 413 (1988b).
Antal, M. and Varhegyi, G., “Cellulose pyrolysis kinetics: the current state of knowledge,”Ind. Eng. Chem. Res.,34, 703 (1995).
Antal, M. J., Varheyi, G. and Jakab, M., “Cellulose pyrolysis kinetics: revisited,”Ind. Eng. Chem. Res.,7, 1267 (1998).
Bigger, S., Scheirs, J. and Camino, G., “An investigation of the kinetics of cellulose degradation under non-isothermal conditions,”Polym. Degrade. Stab.,62, 33 (1998).
Blasi, C., “Numerical simulation of cellulose pyrolysis,”Biomass Bioenerg.,7, 87 (1994).
Blasi, C. D., “Kinetic and heat transfer control in the slow and flash pyrolysis of solids,”Ind. Eng. Chem. Res.,35, 37 (1996).
Bradbury, A., Sakai, Y. and Shafizadeh, F., “Kinetic model for pyrolysis of cellulose,”J. Appl. Polym. Sci.,23, 3271 (1979).
Broido, A.,Kinetics of solid-phase cellulose pyrolysis, Thermal uses and properties of carbohydrates and lignins, Shafizadeh, F., Sarkanen, K. and Tillman, D., eds., Academic Press, New York (1976).
Conesa, J., Caballero, J., Marcilla, A. and Font, R., “Analysis of different kinetic models in the dynamic pyrolysis of cellylose,”Thermochim. Acta.,254, 175 (1995).
Diebold, J., “A unified, global model for the pyrolysis of cellulose,”Biomass Bioenerg.,7, 75 (1994).
Gronli, M., Antal, M. and Varhegyi, G. X., “Round-robin study of cellulose pyrolysis kinetics by thermogravimetry,”Ind. Eng. Chem. Res.,38, 2238 (1999).
Eom, Y., Kim, S., Kim, S. S. and Chung, S. H., “Using the peak property method to estimate apparent kinetic parameters of cellulose pyrolysis reaction,”Submitted for possible publication to Chem. Eng. J. (2005).
Kwon, T. W., Kim, S. D. and Fung, D. P. C., “Reaction kinetics of Char-CO2 gasification,”Fuel,67, 530 (1988).
Halikia, I., Neou-Syngouna, P. and Kolitsa, D., “Isothermal kinetic analysis of the thermal decomposition of magnesium hydroxide using thermogravimetric data,”Thermochim. Acta,320, 75 (1998).
Liliedahl, T. and Sjostrom, K., “Heat transfer controlled pyrolysis kinetics of a biomass slab, rod or sphere,”Biomass Bioenerg.,15, 503 (1998).
Maciejewski, M.,Computational aspects of kinetic analysis. Part B: The ICTAC Kinetic Project — the decomposition kinetics of calcium carbonate revisited, or some tips on survival in the kinetic minefield (2000).
Pyle, D. L. and Zaror, C. A., “Heat transfer and kinetics in the low temperature pyrolysis of solids,”Chem. Eng. Sci.,39, 147 (1984).
Reactions in the Solid State. Comprehensive Chemical Kinetics, Bamford, C. H. and Tipper, C. F.H., eds., Vol. 22. Elsevier, Amsterdam (1980).
Reynolds, J. G. and Burnham, A. K., “Pyrolysi0s decomposition kinetics of cellulose-based materials by constant heating rate micropyrolysis,”Energ. Fuel,11, 88 (1997).
Rodante, F., Vecchio, S. and Tomassetti, M., “Kinetic analysis of thermal decomposition for penicillin sodium salts Model-fitting and model-free methods,”J. Pharm. Biomed. Anal.,29, 1031 (2002).
Tanaka, H., “Thermal analysis and kinetics of solid state reactions,”Thermochim. Acta.,267. 29 (1995).
Varhegyi, G. and Antal, M., “Kinetics of the thermal decomposition of cellulose, hemicellulose, and sugar cane bagasse,”Energ. Fuel,3, 329 (1989).
Varhegyi, G., Antal, M., Jakab, E. and Szabo, P., “Kinetic modeling of biomass pyrolysis,”J. Anal. Appl. Pyrol.,42, 73 (1997).
Varhegyi, G., Jakab, E. and Antal, M., “Is the broido-shafizadeh model for cellulose pyrolysis true?,”Energ. Fuel,8, 1345 (1994).
Vyazovkin, S. and Wight, S. A., “Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data,”Thermochim. Acta.,340–341, 53 (1999).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, S., Eom, Y. Estimation of kinetic triplet of cellulose pyrolysis reaction from isothermal kinetic results. Korean J. Chem. Eng. 23, 409–414 (2006). https://doi.org/10.1007/BF02706742
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02706742