Abstract
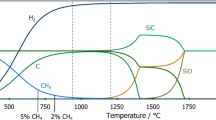
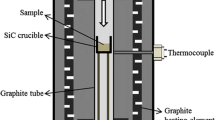
The kinetics and mechanism of reaction between SiO2 and SiC are studied. The reaction rate is shown to be limited by the carbon diffusion from the SiC bulk to the SiC/SiO2 interface, where carbon reacts with the SiO and oxygen resulting from SiO2 decomposition. Equations are presented which describe the time variation of SiC conversion and the temperature variation of the carbon diffusivity in SiC.
Similar content being viewed by others
References
Hong, J.D., Hon, M.H., and Davis, R.F., Self Diffusion in Aplha- and Beta-Silicon Carbide,J. Ceram., 1979, vol. 5, pp. 155–160.
Shei, A., Chemistry of Ferrosilicon Production,Norw. J. Chem., Mining Metall., 1967, vol. 27, no. 8/9, pp. 152–158.
Gel’d, P.V. and Esin, O.A., inIssledovaniya pri vysokikh temperaturakh (High-Temperature Investigation Techniques), Moscow: Nauka, 1967, p. 484.
Serov, G.V., Mizin, V.G., Tolstoguzov, N.V.,et al., Sovershenstvovanie proizvodstva ferrosilitsiya na Kuznetskom zavode ferrosplavov (Developments in the Ferrosilicon Production at the Kuznetsk Ferroalloy Works), Kemerovo, 1969, issue 2, p. 105.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Khrushchev, M.S. Kinetics and mechanism of reaction between silicon carbide and silica. Inorg Mater 36, 462–464 (2000). https://doi.org/10.1007/BF02758048
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02758048