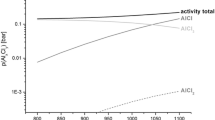
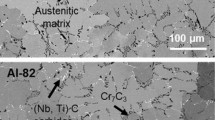
Abstract
The development of continuous Al2O3 scales on alloys via selective oxidation is an extremely effective approach to obtain high-temperature oxidation resistance. Depending on the alloy system, it may be necessary to optimize the selective oxidation process. For all alloy systems, improvements in oxidation resistance can be obtained by optimizing the type and concentrations of reactive elements and by controlling the concentration of sulfur.
Similar content being viewed by others
References
G.C. Wood, Oxid. Metals, 2 (1970), p. 11.
G.C. Wood, Oxidation of Metals and Alloys (Metals Park, OH: ASM, 1971), p. 201.
C.S. Giggins and F.S. Pettit, J. Electrochem. Soc., 118 (1971), p. 1782.
B.H. Kear et al., Oxid. Metals, 3 (1971), p. 557.
G.R. Wallwork and A.Z. Hed, Oxid. Metals, 3 (1971), p. 171.
C.A. Barrett and C.E. Lowell, Oxid. Metals, 11 (1977), p. 199.
J.L. Smialek and G.H. Meier, “High Temperature Oxidation, ” Superalloys II, ed. C.T. Sims, N.S. Stoloff, W.C. Hagel (New York: John Wiley and Sons, 1987), p. 295.
P. Kofstad,High Temperature Corrosion (New York: Elsevier Applied Science, 1988), pp. 342–378.
A. Rahmel and J. Spencer, Oxid. Metals, 35 (1991), p. 53
K.L. Luthra, Oxid. Metals, 36 (1991), p. 475.
E.J. Feiten and F.S. Pettit, “High Temperature Oxidation Behavior of Directionally Solidified Eutectic Alloys,” Failure Modes in Composites II, ed. James N. Fleck and Richard L. Mehan (Warrendale, PA: TMS, 1974), p. 220.
E.J. Feiten and F.S. Pettit, Oxid. Metals, 10, (1976), p. 189.
T.A. Ramanarayanan, M. Raghavan, and R. Petkovic-Luton, J. Electrochem. Soc., 131 (1984), p. 923.
J.L. Smialek and R. Gibala, “Diffusion Processes in A12O3 Scales: Void Growth, Grain Growth and Scale Growth,” High Temperature Corrosion, ed. R.A. Rapp (Houston, TX: National Assoc. of Corrosion Engineers, 1983), p. 274.
J.K. Tien and F.S. Pettit, Met. Trans., 3 (1972), p. 1587.
H.J. Grabke, D. Wiemer, and H. Viefhaus, Appl. Surf. Sci 47 (1991), p. 243.
C. Wagner, Corr. Sci., 5 (1965), p. 751.
C.S. Giggins and F.S. Pettit, Oxid. Metals, 14, (1980), p. 363.
N.S. Choudhury, H.C. Graham, and J.W. Hinze, Properties of High Temperature Alloys, ed. Z.A. Foroulis and F.S. Pettit (Pennington, NJ: Electrochemical Society, 1976), p. 668.
J. Rakowski, Masters thesis, University of Pittsburgh 1994.
C.S. Giggins and F.S. Pettit, TMS-AIME 245, (New York-TMS-AIME, 1969), p. 2509.
M.W. Brumm, H.J. Grabke, and B. Wagemaum, Corr. Sci., 36 (1994), p. 37.
D.P. Whittle and J. Stringer, Phil. Trans. Roy. Soc Lond A295 (1980), p. 309.
F.S. Pettit, “What Are the Effects of Oxide Dispersions in Cr and Al-Containing Alloys on the Kinetics, Growth Direction, Mode of Transport, and Adhesion of the Scale?” AGARD Proceedings No. 120 on High Temperature of Aerospace Alloys (Lyngby, Denmark: Technical Editing and Reproductions, Ltd., Harford House, 1972).
J. Stringer, Met. Rev., 11 (1966), p. 113.
E.J. Feiten, J. Electrochem. Soc., 108 (1961), p. 490.
F.A. Golightly, F.H. Stott, and G.C. Wood, Oxid. Met., 10 (1976), p. 163.
H. Pfeiffer, Werkst. Korros., 8 (1957), p. 574.
J.E. McDonald and J.G. Eberhardt, Trans. TMS-AIME, 233 (1965), p. 512.
J.E. Antill and K.A. Peakall, J. Iron & Steel Inst., 205 (1967) p. 1136.
A.W. Funkenbresch, J.G. Smeggil, and N.S. Bornestein, Met. Trans., 16A, (1985), p. 1164.
J.S. Smeggil, A.W. Funkenbresch, and N.S. Bornestein, Met. Trans., 17A, (1986), p. 923.
B.K. Tubbs and J.L. Smialek, “Effect of Sulfur Removal on Scale Adhesion to PW 1480,” Symposium on Corrosion and Particle Erosion at High Temperatures, ed. V. Srinivasan and K. Vedula (Warrendale, PA: TMS, 1989), p. 459.
R.V. McVay et al, “Oxidation of Low Sulfur Single Crystal Nickel-Base Superalloys,” Superalloys 1992, ed. S.D. Antolovich et al. (Warrendale, PA: TMS, 1992), p. 807.
K. Ohmura, et al., “Effect of Lanthanoid on Oxidation Behavior of Fe-Cr-Al Foil,” High Temperature Corrosion of Advanced Materials and Protective Coatings, ed. Y. Saito, B. Onay, and T. Maruyama (New York: North Holland Publishers, 1992), p. 167.
M.C. Stasik et al., Scripta Met. et Mater., 31, (1994); p. 1645.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Birks, N., Meier, G.H. & Pettit, F.S. Forming continuous alumina scales to protect superalloys. JOM 46, 42–46 (1994). https://doi.org/10.1007/BF03222664
Issue Date:
DOI: https://doi.org/10.1007/BF03222664