Abstract
In mammals, the adipose organ is a multi-depot organ made of two tissue types, the white and brown adipose tissues, which collaborate in partitioning the energy contained in lipids between thermogenesis and the other metabolic functions. It consists of several sc and visceral depots. Some areas of these depots are brown and correspond to brown adipose tissue, while many are white and correspond to white adipose tissue. White areas contain a variable amount of brown adipocytes and their number varies with age, strain and environmental conditions. Brown and white adipocyte are morphologically different. At light microscopy level, brown adipocytes have cytoplasmic lipids arranged as numerous small droplets (multilocularity), while white adipocytes have cytoplasmic lipids arranged in a unique vacuole (unilocularity). Ultrastructurally, brown adipocytes have numerous big mitochondria packed with cristae and containing the thermogenic uncoupling protein 1 (UCP1). In vivo and in vitro studies have shown that the differentiation process of brown and white adipocytes shows distinctive features. Nevertheless, the origin of the adipocyte precursor is still unknown. Recent data have stressed the plasticity of the adipose organ in adult animals. Indeed, under peculiar conditions fully differentiated, white adipocytes can transdifferentiate into brown adipocytes, and viceversa. The ability of the adipose organ to interconvert its main cytotypes in order to meet changing metabolic needs is highly pertinent to the physiopathology of obesity and related to therapeutic strategies.
Similar content being viewed by others
References
Cinti S. The adipose organ. Kurtis Ed., Milano, 1999.
Sbarbati A., Morroni M., Zancanaro C., Cinti S. Rat interscapular brown adipose tissue at different ages: a morphometric study. Int. J. Obes. 1991, 15: 581–587.
Bukowiecki L.J. Lipid metabolism in brown adipose tissue. In: Trayhurn P., Nicholls D.G. (Eds.), Brown adipose tissue. Edward Arnold, London, 1986, p. 105.
Néchad M. Structure and development of brown adipose tissue. In: Trayhurn P., Nicholls D.G. (Eds.), Brown adipose tissue. Edward Arnold, London, 1986, p. 1.
Foster D.O., Depocas F., Zaror-Beherens G. Unilaterality of the sympathetic innervation of each pad of rat interscapular brown adipose tissue. Can. J. Physiol. Pharmacol. 1982, 60: 107–113.
Nnodim J.O., Lever J.D. Neural and vascular provisions of rat interscapular brown adipose tissue. Am. J. Anat. 1988, 182: 283–293.
Norman D., Mukherjee S., Symons D., Jung R.T., Lever J.D. Neuropeptides in interscapular and perirenal brown adipose tissue in the rat: a plurality of innervation. J. Neurocytol. 1988, 17: 305–311.
De Matteis R., Ricquier D., Cinti S. TH-, NPY-, SP-, and CGRP-immunoreactive nerves in interscapular brown adipose tissue of adult rats acclimated at different temperatures: an immunohistochemical study. J. Neurocytol. 1998, 27: 877–886.
Cannon B., Nedergaard J., Lundberg J.M., Hokfelt T., Terenius L., Goldstein M. Neuropeptide tyrosine (NPY) is co-stored with noradrenaline in vascular but not in parenchymal sympathetic nerves of brown adipose tissue. Exp. Cell Res. 1986, 164: 546–550.
Himms-Hagen J. Brown adipose tissue thermogenesis: interdisciplinary studies. FASEB J. 1990, 4: 2890–2898.
Nisoli E., Tonello C., Benarese M., Liberini P., Carruba M.O. Expression of nerve growth factor in brown adipose tissue: implications for thermogenesis and obesity. Endocrinology 1996, 137: 495–503.
Bartness T.J., Bamshad M. Innervation of mammalian white adipose tissue: implications for the regulation of total body fat. Am. J. Physiol. 1998, 275: R1399–R1411.
Napolitano L., Fawcett D. The fine structure of brown adipose tissue in the newborn mouse and rat. J. Biophys. Biochem. Cytol. 1958, 4: 685–690.
Napolitano L. The differentiation of white adipose tissue: an electron microscopic study. J. Cell Biol. 1963, 18: 663–673.
Afzelius B.A. Brown adipose tissue: its gross anatomy, histology, and cytology. In: Lindberg O. (Ed.), Brown adipose tissue. Elsevier, Amsterdam, 1970, p. 1.
Zhang Y.Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372: 425–432.
Friedman J.M., Halaas J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395: 763–770.
Trayhurn P., Hoggard N., Rayner D.V. White adipose tissue as a secretory and endocrine organ: leptin and other secreted proteins In: Klaus S. (Ed.), Adipose tissues. Eurekah.com/ Landes Bioscience, Georgetown, 2001, p. 158.
Migliorini R.H., Garofalo M.A., Kettelhut I.C. Increased sympathetic activity in rat white adipose tissue during prolonged fasting. Am. J. Physiol. 1997, 272: R656–R661.
Bartness T.J., Demas G.E., Song C.K. Central nervous system innervation of white adipose tissue. In: Klaus S. (Ed.), Adipose tissues. Eurekah.com/Landes Bioscience, Georgetown, 2001, p. 116.
Hodgson A.J., Wilkinson C., Abolhasan P., Llewellyn-Smith I.J. Differential control of lipolysis by sympathetic nerves. Int. J. Obes. 2001, 25: O27 (Abstract).
Cannon B., Hedin A., Nedergaard J. Exclusive occurrence of thermogenin antigen in brown adipose tissue. FEBS Lett. 1982, 150: 129–132.
Cinti S., Zancanaro C., Sbarbati A. et al. Immunoelectron microscopical identification of the uncoupling protein in brown adipose tissue mitochondria. Biol. Cell 1989, 67: 359–362.
Klaus S., Casteilla L., Bouillaud F., Ricquier D. The uncoupling protein UCP: a membraneous mitochondrial ion carrier exclusively expressed in brown adipose tissue. Int. J. Biochem. 1991, 23: 791–801.
Ricquier D., Casteilla L., Bouillaud F. Molecular studies of the uncoupling protein. FASEB J. 1991, 5: 2237–2242.
Garruti G., Ricquier D. Analysis of uncoupling protein and its mRNA in adipose tissue deposits of adult humans. Int. J. Obes. 1992, 16: 383–390.
Klaus S. Brown adipose tissue: thermogenic function and its physiological regulation. In: Klaus S. (Ed.), Adipose tissues. Eurekah.com/Landes Bioscience, Georgetown, 2001, p. 56.
Klaus S. Brown adipocyte differentiation and function in energy metabolism. In: Klaus S. (Ed.), Adipose tissues. Eurekah. com/Landes Bioscience, Georgetown, 2001, p. 82.
Himms-Hagen J. Brown adipose tissue and cold-acclimation. In: Trayhurn P., Nicholls D.G. (Eds.), Brown adipose tissue. Edward Arnold, London, 1986, p. 214.
Girardier L., Seydoux J. Neural control of brown adipose tissue. In: Trayhurn P., Nicholls D.G. (Eds.), Brown adipose tissue. Edward Arnold, London, 1986, p. 122.
Strosberg A.D., Pietri-Rouxel F. Function and regulation of the beta 3-adrenoceptor. Trends Pharmacol. Sci. 1996, 17: 373–381.
Rothwell N.J., Stock M.J. A role for brown adipose tissue in diet-induced thermogenesis. Nature 1979, 281: 31–33.
Lowell B.B., Susulic V., Hamann A. et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 1993, 366: 740–742.
Enerback S., Jacobsson A., Simpson E.M., et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 1997, 387: 90–94.
Matthias A., Ohlson K.B., Fredriksson J.M., Jacobsson A., Nedergaard J., Cannon B. Thermogenic responses in brown fat cells are fully UCP1-dependent. UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty scidinduced thermogenesis. J. Biol. Chem. 2000, 275: 25073–25081.
Kopecky J., Clarke J., Enerback B., Spiegelman B., Kozak L.P. Ectopic expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J. Clin. Inv. 1995, 96: 2914–2923.
Rossmeisl M., Barbatelli G., Flachs P. et al. Expression of the uncoupling protein 1 from the aP2 gene promoter stimulates mitochondrial biogenesis in unilocular adipocytes in vivo. Eur. J. Biochem. 2002, 269: 19–28.
Spiegelman B.M., Choy L., Hotamisligil G.S., Graves R.A., Tontonoz P. Regulation of adipocyte gene expression in differentiation and syndromes of obesity/diabetes. J. Biol. Chem. 1993, 268: 6823–6826.
Gregoire F.M., Smas C.M., Sul H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78: 783–809.
Rosen E.D., Walkey C.J., Puigserver P., Spiegelman B.M. Transcriptional regulation of adipogenesis. Genes Dev. 2000, 14: 1293–1307.
Ailhaud G. Development of white adipose tissue and adipocyte differentiation. In: Klaus S. (Ed.), Adipose tissues. Eurekah.com/Landes Bioscience, Georgetown, 2001, p. 27.
Zancanaro C., Carnielli V.G., Moretti C., Benati D., Gamba P. An ultrastructural study of brown adipose tissue in preterm human new-borns. Tissue Cell 1995, 27: 339–348.
Kim H.S., Hausman G.J., Hausman D.B., Martin R.J., Dean R.G. The expression of peroxisome proliferator-activated receptor gamma in pig fetal tissue and primary stromalvascular cultures. Obes. Res. 2000, 8: 83–88.
Hauner H., Skurk T. Adipose tissue pathology in human obesity. In: Klaus S. (Ed.), Adipose tissues. Eurekah.com/ Landes Bioscience, Georgetown, 2001, p. 27.
Barnard T., Skàla J. The development of brown adipose tissue. In: Lindberg O. (Ed.), Brown adipose tissue. Elsevier, New York, 1970, p. 33.
HoustekJ.KopeckyJ.RychterZ.SoukupT.Uncouplingproteininembryonicbrownadiposetissue—existenceofnthermogenicandthermogenicmitochondria.Biochim.Biophys.Acta198893519–25
Van R.L.R., Bayliss C.E., Roncari D.A.K. Cytological and enzymological characterization of adult human adipocyte precursors in culture. J. Clin. Invest. 1976, 58: 699–704.
Slavin B.G. Fine structural studies on white adipocyte differentiation. Anat. Rec. 1979, 195: 63–72.
Cinti S., Cigolini M., Bosello O., Björntorp P. A morphological study of the adipocyte precursor. J. Submicrosc. Cytol. Pathol. 1984, 16: 243–251.
Barbatelli G., Morroni M., Vinesi P., Cinti S., Michetti F. S- 100 protein in rat brown adipose tissue under different functional conditions: a morphological, immunocytochemical, and immunochemical study. Exp. Cell Res. 1993, 208: 226–231.
Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int. J. Biochem. Cell. Biol. 2001, 33: 637–668.
Prins J.B., O’Rahilly S. Regulation of adipose cell number in man. Clin. Sci. 1997, 92: 3–11.
Hausman D.B., DiGirolamo M., Bartness T.J., Hausman G.J., Martin R.J. The biology of white adipocyte proliferation. Obes. Rev. 2001, 2: 239–254.
Wajchenberg B.L. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr. Rev. 2000, 21: 697–738.
Carey D.G., Nguyen T.V., Campbell L.V., Chisholm D.J., Kelly P. Genetic influences on central abdominal fat: a twin study. Int. J. Obes. 1996, 20: 722–726.
Björntorp P. Size, number and function of adipose tissue cells in human obesity. Horm. Metab. Res. 1974, 4: 77–83.
Di Girolamo M., Fine J.B., Tagra K., Rossmanith R. Qualitative regional differences in adipose tissue growth and cellularity in male Wistar rats fed ad libitum. Am. J. Physiol. 1998, 274: R1460–R1467.
Lemmonier D. Effect of age, sex, and site on the cellularity of the adipose tissue in mice and rats rendered obese by a high fat diet. J. Clin. Invest. 1972, 51: 2907–2915.
Faust I.M., Johnson P.R., Stern J.S., Hirsch J. Diet-induced adipocyte number increase in adult rats: a new model of obesity. Am. J. Physiol. 1978, 235: E279–E286.
Björntorp B. Adipose tissue distribution and function. Int. J. Obes. 1991, 15: 67–81.
Ahima R.S., Flier J.S. Adipose tissue as an endocrine organ. Trends Endocrinol. Metab. 2000, 11: 327–332.
Johnson P.R., Hirsch J. Cellularity of adipose depots in six strains of genetically obese mice. J. Lipid Res. 1972, 13: 2–11.
Machinal-Quélin F., Dieudonné M.N., Leneveu M.C., Giudicelli Y., Pecquery R. Adipogenic effects of leptin are mediated by functional leptin receptors in rat preadipocytes. Int. J. Obes. 2001 25: S29 (Abstract).
De Matteis R., Dashtipour K., Ognibene A., Cinti S. Localization of leptin receptor splice variants in mouse peripheral tissues by immunohistochemistry. Proc. Nutr. Soc. 1998, 57: 441–448.
Imai T., Jiang M., Chambon P., Metzger D. Impaired adipogenesis and lipolysis in the mouse upon selective ablation of the retinoid X receptor alpha mediated by a tamoxifeninducible chimeric Cre recombinase (Cre-ERT2) in adipocytes. Proc. Natl. Acad. Sci. USA 2001, 98: 224–228.
Valet P., Grujic D., Wade J. et al. Expression of human α2- adrenergic receptors in adipose tissue of β3-adrenergic receptor-deficient mice promotes diet-induced obesity. J. Biol. Chem. 2000, 275: 34797–34802.
Valet P., Pagès C., Jeanneton O. et al. Alpha2-adrenergic receptor-mediated release of lysophosphatidic acid by adipocytes. J. Clin. Inv. 1998, 101: 1431–1438.
Bulbarelli A., Morroni M., Nisoli E., Carruba M.O., Cinti S. Brown and white adipocytes are differently prone to apoptotic stimuli. Int. J. Obes. 2001, 25: S11 (Abstract).
Cheng A.Y.M., Deitel M., Ronkari A.K. Cytological and enzymological characterization of adult human adipocyte precursors in culture. J. Clin. Invest. 1976, 58: 699–704.
Van R.L.R., Ronkari A.K. Complete differentiation of adipocyte precursors. Cell Tissue Res. 1978, 195: 317–329.
Petruschke T.H., Hauner H. Tumor necrosis factor-a prevents the differentiation of human adipocyte precursor cells and causes delipidation of newly developed fat cells. J. Clin. Endocrinol. Metab. 1993, 76: 742–747.
Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity- linked insulin resistance. Science 1993, 259: 87–91.
Nisoli E., Briscini L., Giordano A. et al. Tumor necrosis factor a mediates apoptosis of brown adipocytes and defective brown adipocyte function in obesity. Proc. Natl. Acad. Sci. USA 2000, 97: 8033–8038.
Pagano C., Calcagno A., Dorigo A. et al. Administration of all-trans retinoic acid inhibits adipose tissue deposition and induces apoptosis of adipose cells in lean and obese (fa/fa) zucker rats. Int. J. Obes. 2001, 25 (Suppl. 2): S3 (Abstract).
Geloen A., Collet A.F., Bukowiecki L.J. Role of sympathetic innervation in brown adipocyte proliferation. Am. J. Physiol. 1992, 263: R1176–R1181.
Morroni M., Barbatelli G., Zingaretti M.C., Cinti S. Immunohistochemical, ultrastructural and morphometric evidence for brown adipose tissue recruitment due to cold acclimation in old rats. Int. J. Obes. 1995, 19: 126–131.
Huttunen P., Hirvonen J., Kinnula V. The occurrence of brown adipose tissue in outdoor workers. Eur. J. Appl. Physiol. 1981, 46: 339–345.
Kortelainen M.-L., Pelletier G., Ricquier D., Bukowiecki L.J. Immunohistochemical detection of human brown adipose tissue uncoupling protein in an autopsy series. J. Histochem. Cytochem. 1993, 41: 759–764.
Ricquier D., Néchad M., Mory G. Ultrastructural and biochemical characterization of human brown adipose tissue in pheochromocytoma. J. Clin. Endocrinol. Metab. 1982, 54: 803–807.
Bouillaud F., Combes-George M., Ricquier D. Mitochondria of human brown adipose tissue contain a 32000- Mr uncoupling protein. Biosci. Rep. 1983, 3: 775–780.
Lean M.E.J., James W.P.T., Jennings G., Trayhurn P. Brown adipose tissue in patients with pheochromocytoma. Int. J. Obes. 1986, 10: 219–227.
Loncar D. Convertible adipose tissue in mice. Cell Tissue Res. 1991, 266: 149–161.
Giordano A., Coppari R., Castellucci M., Cinti S. Sema3a is produced by brown adipocytes and its secretion is reduced following cold acclimation. J. Neurocytol. 2001, 30: 5–10.
Cinti S., Frederich R.C., Zingaretti M.C., De Matteis R., Flier J.S., Lowell B.B. Immunohistochemical localization of leptin and uncoupling protein in white and brown adipose tissue. Endocrinology 1997, 138: 797–804.
Cancello R., Zingaretti M.C., Sarzani R., Ricquier D., Cinti S. Leptin and UCP1 genes are reciprocally regulated in brown adipose tissue. Endocrinology 1998, 139: 4747–4750.
Champigny O., Ricquier D., Blondel O., Mayer R.M., Briscoe M.G., Halloway B.R. β3-adrenergic receptor stimulation restores message and expression of brown-fat mitochondrial uncoupling protein in adult dogs. Proc. Natl. Acad. Sci. USA 1991, 88: 10774–10777.
Cousin B., Cinti S., Morroni M. et al. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J. Cell Sci. 1992, 103: 931–942.
Himms-Hagen J., Cui J., Danforth E. et al. Effect of CL- 316,243, a thermogenic β3-agonist, on energy balance and brown and white adipose tissues in rats. Am. J. Physiol. 1994, 266: R1371–R1382.
Collins S., Daniel K.W., Petro E., Surwitt R.S. Strain-specific response to β3-adrenergic receptor agonist treatment on diet-induced obesity in mice. Endocrinology 1997, 138: 405–413.
Ghorbani M., Claus T.H., Himms-Hagen J. Hypertrophy of brown adipocytes in brown and white adipose tissues and reversal of diet-induced obesity in rats treated with a beta3-adrenoceptor agonist. Biochem. Pharmacol. 1997, 54: 121–131.
Ghorbani M., Himms-Hagen J. Appearance of brown adipocytes in white adipose tissue during CL 316,243-induced reversal of besity and diabetes in Zucker fa/fa rats. Int. J. Obes. 1997, 21: 465–475.
Guerra C., Koza R.A., Yamashita H., Walsh K., Kozack L.P. Emergence of brown adipocytes in white fat in mice is under genetic control. J. Clin. Invest. 1998, 102: 412–420.
Geloen A., Collet A.F., Guay G., Bukowiecki L.J. Beta adrenergic stimulation of brown adipocyte proliferation. Am. J. Physiol. 1988, 254: C175–C182.
Geloen A., Collet A.F., Guay G., Bukowiecki L.J. In vivo differentiation of brown adipocytes in adult mice: an electron microscopic study. Am. J. Anat. 1990, 188: 366–372.
Goglia F., Geloen A., Lanni A., Minaire Y., Bukowiecki L.J. Morphometric-stereologic analysis of brown adipocyte differentiation in adult mice. Am. J. Physiol. 1992, 262: C1018–C1023.
Bronnikov G., Houstek J., Nedergaard J. Beta-adrenergic, cAMP-mediated stimulation of proliferation of brown fat cells in primary culture. Mediation via beta 1 but not via beta 3 adrenoceptors. J. Biol. Chem. 1992, 267: 2006–2013.
Bronnikov G., Bengtsson T., Kramarova L., Golozoubova V., Cannon B., Nedergaard J. Beta1 to beta3 switch in control of cyclic adenosine monophosphate during brown adipocyte development explains distinct beta-adrenoceptor subtype mediation of proliferation and differentiation. Endocrinology 1999, 140: 4185–4197.
Granneman J.G., Lahners K.N., Chaudhry A. Molecular cloning and expression of the rat beta 3-adrenergic receptor. Mol. Pharmacol. 1991, 40: 895–899.
Chamberlain P.D., Jennings K.H., Paul F. et al. The tissue distribution of the human beta3-adrenoceptor studied using a monoclonal antibody: direct evidence of the beta3- adrenoceptor in human adipose tissue, atrium and skeletal muscle. Int. J. Obes. 1999, 23: 1057–1065.
Himms-Hagen J., Melnyk A., Zingaretti M.C., Ceresi E., Barbatelli G., Cinti S. Multilocular fat cells in WAT of CL- 316243-treated rats derive directly from white adipocytes. Am. J. Physiol. 2000, 279: C670–C681.
Author information
Authors and Affiliations
Corresponding author
Additional information
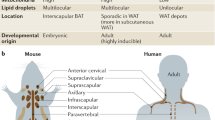
In this review, all descriptions will be intended of the adipose organ of rats and mice if not differently specified.
Rights and permissions
About this article
Cite this article
Cinti, S. Adipocyte differentiation and transdifferentiation: Plasticity of the adipose organ. J Endocrinol Invest 25, 823–835 (2002). https://doi.org/10.1007/BF03344046
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03344046