Abstract
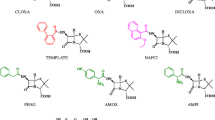
In the present work, the synthesis and characterisation of a molecularly imprinted polymer (MIP) by precipitation polymerisation using tetracycline (TC) as a template molecule, methacrylic acid as a functional monomer, ethylene glycol dimethacrylate as a cross-linker, persulphate as an initiator, and methanol as a porogen solvent is described. The molecular recognition properties and selectivity of MIPs against four TCs [(TC), oxytetracycline (OT), chlortetracycline (CT), and doxycycline (DT)] were evaluated, and the results demonstrated high selectivity for four TCs in milk samples without sample treatment. Under the optimal synthesis conditions (TC/methacrylic acid/ethylene glycol dimethacrylate ratio of 1.0:7.0:50.0), the percent removal obtained was 81.83% for TC, 95.47% for OT, 96.44% for CT, and 93.25% for DT using 1.0 mL of the sample, 20 mg of the sorbent, a pH of 6.0, and 15 min of contact time. One of the tested samples was positive for the presence of OT, with a concentration of 19.83 μg L−1, ensuring the complete removal of TC using the proposed method. The method provided significant data regarding the development of efficient and selective materials in the removal of several residues and contaminants.











Similar content being viewed by others
References
Guarddon M, Miranda JM, Rodríguez JA, Vázquez BI, Cepeda A, Franco CM (2011) Real-time polymerase chain reaction for the quantitative detection of tetA and tetB bacterial tetracycline resistance genes in food. Int J Food Microbiol 146:284–289. https://doi.org/10.1016/j.ijfoodmicro.2011.02.026
Gu Y, Walker C, Ryan ME, Payne JB, Golub LM (2012) Non-antibacterial tetracycline formulations: clinical applications in dentistry and medicine. J Oral Microbiol 4:1–14. https://doi.org/10.3402/jom.v4i0.19227
Ibarra IS, Rodriguez JA, Miranda J, Vega M, Barrado E (2011) Magnetic solid phase extraction based on phenyl silica adsorbent for the determination of tetracyclines in milk samples by capillary electrophoresis. J Chromatogr A 1218:2196–2202. https://doi.org/10.1016/j.chroma.2011.02.046
Suárez B, Santos B, Simonet BM, Cárdenas S, Varcárcel M (2007) Solid-phase extraction-capillary electrophoresis-mass spectrometry for the determination of tetracyclines residues in surface water by using carbon nanotubes as sorbent material. J Chomatogr A 1175:127–132. https://doi.org/10.1016/j.chroma.2007.10.033
Grenni P, Ancona V, Barra CA (2018) Ecological effects of antibiotics on natural ecosystems: a review. Microchem J 136:25–39. https://doi.org/10.1016/j.microc.2017.02.006
Liu Y, Yang H, Yang S, Hu Q, Cheng H, Liu H, Qiu Y (2013) High-performance liquid chromatography using pressurized liquid extraction for the determination of seven tetracyclines in egg, fish and shrimp. J Chromatogr B 917-918:11–17. https://doi.org/10.1016/j.jchromb.2012.12.036
Mookantsa SOS, Dube S, Nindi MM (2016) Development and application of a dispersive liquid–liquid microextraction method for the determination of tetracyclines in beef by liquid chromatography mass spectrometry. Talanta 148:321–328. https://doi.org/10.1016/j.talanta.2015.11.006
Moudgil P, Bedi JS, Aulakh RS, Gill JPS, Kumar A (2019) Validation of HPLC multi-residue method for determination of fluoroquinolones, tetracycline, sulphonamides and chloramphenicol residues in bovine milk. Food Anal Method 12:338–346. https://doi.org/10.1007/s12161-018-1365-0
Islas G, Rodriguez JA, Perez-Silva I, Miranda JM, Ibarra IS (2018) Solid-phase extraction and large-volume sample staking-capillary electrophoresis for determination of tetracycline residues in milk. J Anal Methods Chem 2018:1–7. https://doi.org/10.1155/2018/5394527
Kemper N (2018) Veterinary antibiotics in the aquatic and terrestrial environment. Ecol Indic 8:1–13 https://doi.org/10.1016/j.ecolind.2007.06.002
Manzetti S, Ghisi R (2014) The environmental release and fate of antibiotics. Mar Pollut Bull 79:7–15. https://doi.org/10.1016/j.marpolbul.2014.01.005
Pérez-Rodríguez M, Pellerano RG, Pezza L, Pezza HR (2018) An overview of the main foodstuff sample preparation technologies for tetracycline residue determination. Talanta 182:1–21. https://doi.org/10.1016/j.talanta.2018.01.058
United States Food and Drugs Administration (FDA) (2017) National Drug Residue Milk Monitoring Program: 2017. Available online: https://www.fda.gov/food/food-compliance-programs/national-drug-residue-milk-monitoring-program (accessed on 10.07.2019)
European Commision Regulation 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off J Eur Union, L15, 1–72
Codex Alimentarius Commision (CAC) (2015) Límites máximos de residuos (LMR) y recomendaciones sobre la gestión de riesgos (RGR) para residuos de medicamentos veterinarios en los alimentos. CAC/MRL 02-2015 1–40
Malik AH, Iyer PK (2017) Conjugated polyelectrolyte based sensitive detection and removal of antibiotics tetracycline from water. ACS Appl Mater Inter 9:4433–4439. https://doi.org/10.1021/acsami.6b13949
Wang T, Pan X, Ben W, Wang J, Hou P, Qiang Z (2017) Adsorptive removal of antibiotics from water using magnetic ion exchange resin. J Environ Sci 52:111–117. https://doi.org/10.1016/j.jes.2016.03.017
Ahmed MB, Zhou JL, Ngo HH, Guo W (2015) Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci Total Environ 532:112–126. https://doi.org/10.1016/j.scitotenv.2015.05.130
Rivera-Utrilla J, Gómez-Pacheco C, Sánchez-Polo M, López-Peñalver JJ, Ocampo-Pérez R (2013) Tetracycline removal from water by adsorption/bioadsorption on activated carbons and sludge-derived adsorbents. J Environ Manag 131:16–24. https://doi.org/10.1016/j.jenvman.2013.09.024
Wang D, Jia F, Wang H, Chen F, Fang Y, Dong W, Zeng G, Li X, Yang Q, Yuan X (2018) Simultaneously efficient adsorption and photocatalytic degradation of tetracycline by Fe-based MOFs. J Colloid Interf Sci 519:273–284. https://doi.org/10.1016/j.jcis.2018.02.067
Lin XH, Aik SXL, Angkasa J, Le Q, Chooi KS, Li SFY (2018) Selective and sensitive sensors based on molecularly imprinted poly(vinylidene fluoride) for determination of pesticides and chemical threat agent simulants. Sensor Actuat B-Chem 258:228–237. https://doi.org/10.1016/j.snb.2017.11.070
Cela-Pérez MC, Lasagabáster A, Abad-López MJ, López-Vilariño JM, Gónzalez-Rodríguez MV (2013) A study of competitive molecular interaction effects on imprinting of molecularly imprinted polymers. Vib Spectrosc 65:74–83. https://doi.org/10.1016/j.vibspec.2012.12.002
Hu Y, Pan J, Zhang K, Lian H, Li G (2013) Novel applications of molecularly-imprinted polymers in sample preparation. TrAc-Trends Anal Chem 43:37–52. https://doi.org/10.1016/j.trac.2012.08.014
Tan F, Sun D, Gao J, Zhao Q, Wang X, Teng F, Quan X, Chen J (2013) Preparation of molecularly imprinted polymer nanoparticles for selective removal of fluoroquinolone antibiotics in aqueous solution. J Hazard Mater 244:750–757. https://doi.org/10.1016/j.jhazmat.2012.11.003
Caro E, Marcé RM, Cormack PAG, Sherrington DC, Borrull F (2006) Novel enrofloxacin imprinted polymer applied to the solid-phase extraction of fluorinated quinolones from urine and tissue samples. Anal Chim Acta 562:145–151. https://doi.org/10.1016/j.aca.2006.01.080
Ebrahimzadeh H, Asgharinezhad AA, Mozzen E, Amini MM, Sadeghi O (2015) A magnetic ion-imprinted polymer for lead(II) determination: a study on the adsorption of lead(II) by beverages. J Food Compos Anal 41:74–80. https://doi.org/10.1016/j.jfca.2015.02.001
Huang W, Kong Y, Yang W, Ni X, Wang N, Lu Y, Xu W (2016) Preparation and characterization of novel thermosensitive magnetic molecularly imprinted polymers for selective recognition of norfloxacin. J Polym Res 23:94. https://doi.org/10.1007/s10965-016-0972-y
Monier M, Abdel-Latif DA (2017) Fabrication of au(III) ion-imprinted polymer based on thiol-modified chitosan. Int J Biol Macromol 105:777–787. https://doi.org/10.1016/j.ijbiomac.2017.07.098
Speltini A, Scalabrini A, Maraschi F, Sturini M, Profumo A (2017) Newest applications of molecularly imprinted polymers for extraction of contaminants from environmental and food matrices: a review. Anal Chim Acta 974:1–26. https://doi.org/10.1016/j.aca.2017.04.042
Puzio K, Claude B, Amalric L, Berho C, Grellet E, Bayoudh S, Nehmé R, Morin P (2014) Molecularly imprinted polymer dedicated to the extraction of glyphosate in natural waters. J Chromatogr A 1361:1–8. https://doi.org/10.1016/j.chroma.2014.07.043
Ahmad AL, Lah NFC, Low SC (2018) Configuration of molecular imprinted polymer for electrochemical atrazine detection. J Polym Res 25:243. https://doi.org/10.1007/s10965-018-1595-2
Sajini T, Gigimol MG, Mathew B (2019) Kinetic and thermodynamic studies of molecularly imprinted polymers for the selective adsorption and specific enantiomeric recognition of D-mandelic acid. J Polym Res 26:88. https://doi.org/10.1007/s10965-019-1746-0
Ashley J, Shahbazi MA, Kant K, Chidambara VA, Wolff A, Bang DD, Sun Y (2017) Molecularly imprinted polymers for sample preparation and biosensing in food analysis: Progress and perspectives. Biosens Bioelectron 91:606–615. https://doi.org/10.1016/j.bios.2017.01.018
Kadhem AJ, Xiang S, Nagel S, Lin C, Fidalgo de Cortalezzi M (2018) Photonic molecularly imprinted polymer film for the detection of testosterone in aqueous samples. Polymer 10:349. https://doi.org/10.3390/polym10040349
Oliveira FM, Segatelli MG, Tarley CRT (2015) Preparation of a new restricted access molecularly imprinted hybrid adsorbent for the extraction of folic acid from milk powder samples. Anal Methods 8:656–665. https://doi.org/10.1039/c5ay02410b
Sánchez-Polo M, Velo-Gala I, López-Peñalver JJ, Rivera-Utrilla J (2015) Molecular imprinted polymer to remove tetracycline from aqueous solutions. Micropor Mesopor Mat 203:32–40. https://doi.org/10.1016/j.micromeso.2014.10.022
Lee SH, Doong RA (2012) Adsorption and selective recognition of 17ß-estradiol by molecularly imprinted polymers. J Polym Res 19:9939. https://doi.org/10.1007/s10965-012-9939-9
Asman S, Mohamad S, Sarih NM (2005) Exploiting β-cyclodextrin in molecular imprinting for achieving recognition of benzylparaben in aqueous media. Int J Mol Sci 16:3656–3676. https://doi.org/10.3390/ijms16023656
Ashkenani H, Taher MA (2012) Selective voltammetric determination of cu(II) on multiwalled carbon nanotubes and nano-porous cu-ion imprinted polymer. J Electroanal Chem 683:80–87. https://doi.org/10.1016/j.jelechem.2012.08.010
Cai W, Gupta RB (2004) Molecularly-imprinted polymers selective for tetracycline binding. Sep Purif Technol 35:215–221. https://doi.org/10.1016/S1383-5866(03)00143-6
Regal P, Díaz-Bao M, Barreiro R, Cepeda A, Fente C (2012) Application of molecularly imprinted polymers in food analysis: clean-up and chromatographic improvements. Cent Eur J Chem 10:766–784. https://doi.org/10.2478/s11532-012-0016-3
Konicki W, Aleksandrak M, Mijowska E (2017) Equilibrium, kinetic and thermodynamic studies on adsoption of cationic dyes from aqueous solutions using grapheme oxide. Chem Eng Res Des 123:35–49. https://doi.org/10.1016/j.cherd.2017.03.036
Konicki W, Aleksandrak M, Monszyński D, Mijowska E (2017) Adsoption of anionic azo-dyes from aqueous solutions onto grapheme oxide: equilibrium, kinetic and thermodynamic studies. J Colloid Interf Sci 496:188–200. https://doi.org/10.1016/j.jcis.2017.02.031
Khoo WC, Kamaruzaman S, Lim HN, Jamil SNAM, Yahaya N (2019) Synthesis and characterization of graphene oxide-molecularly imprinted polymer for Neopterin adsorption study. J Polym Res 26:184. https://doi.org/10.1007/s10965-019-1847-9
Song X, Niu Y, Zhang P, Zhang C, Zhang Z, Zhu Y, Qu R (2017) Removal of co(II) from fuel ethanol by silica-gel supported PAMAM dendrimers: combined experimental and theoretical study. Fuel 199:91–101. https://doi.org/10.1016/j.fuel.2017.02.076
Jiang W, Beloglava NV, Wang Z, Jiang H, Wen K, de Saeger S, Luo P, Wu Y, Shen J (2015) Development of a multiplex flow-through immunoaffinity chromatography test for the on-site of 14 sulfonamide and 13 quinolone residues in milk. Biosens Bioelectron 66:124–128. https://doi.org/10.1016/j.bios.2014.11.004
Zhang YD, Zheng N, Han RW, Zheng BQ, Yu ZN, Li SL, Zheng SS, Wang JQ (2014) Occurrence of tetracyclines, sulfonamides, sulfamethazine and quinolones in pasteurized milk and UHT milk in China’s market. Food Control 36:238–242. https://doi.org/10.1016/j.foodcont.2013.08.012
Gaucheron F (2005) The minerals of milk. Reproduction Nutr Develop 45:473–483. https://doi.org/10.1051/rnd:2005030
Aggarwal S, Singh RY, Sing G, Sharma R (2016) Synthesis and characterization of oxytetracycline imprinted magnetic polymer for application in food. Appl Nanosci 6:209–214. https://doi.org/10.1007/s13204-015-0437-3
Dai J, Pan J, Xu L, Li X, Zhou Z, Zhang R, Yan Y (2012) Preparation of molecularly imprinted nanoparticles with superparamagnetic susceptibility through atom transfer radical emulsion polymerization for the selective recognition of tetracycline from aqueous medium. J Hazard Mater 205-206:179–188. https://doi.org/10.1016/j.jhazmat.2011.12.056
Kong J, Wang Y, Nie C, Ran D, Jia X (2012) Preparation of magnetic mixed-templates molecularly imprinted polymer for the separation of tetracycline antibiotics from egg and honey samples. Anal Methods 4:1005–1011. https://doi.org/10.1039/c2ay05662c
Suedde R, Srichana T, Chuchome T, Kongmark U (2004) Use of a molecularly imprinted polymers from a mixture of tetracycline and its degradation products to procedure affinity membranes for the removal of tetracycline from water. J Chromatogr B 811:191–200. https://doi.org/10.1016/j.jchromb.2004.08.044
Yeşilova E, Osman B, Kara A, Tümay ÖT (2018) Molecularly imprinted particle embedded composite cryogel for selective tetracycline adsorption. Sep Purifi Technol 200:155–163. https://doi.org/10.1016/j.seppur.2018.02.002
Acknowledgements
The authors wish to thank Programa para el Desarrollo Profesional Docente, para el Tipo Superior (PRODEP), Consejo Nacional de Ciencia y Tecnología (CONACyT) (SNI distinction as research membership and scholarships), for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aguilar, J.F., Miranda, J.M., Rodriguez, J.A. et al. Selective removal of tetracycline residue in milk samples using a molecularly imprinted polymer. J Polym Res 27, 176 (2020). https://doi.org/10.1007/s10965-020-02139-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-020-02139-9