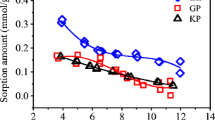
Abstract
Hematite and goethite nanoparticles were used as model minerals to investigate their aggregation kinetics under soil environmental conditions in the present study. The hydrodynamic diameters of hematite and goethite nanoparticles were 34.4 and 66.3 nm, respectively. The positive surface charges and zeta potential values for goethite were higher than for hematite. The effective diameter for goethite was much larger than for hematite due to anisotropic sticking of needle-shaped goethite during aggregation. Moreover, the critical coagulation concentration (CCC) values of nanoparticles in solutions of NaNO3, NaCl, NaF, and Na2SO4 were 79.2, 75.0, 7.8, and 0.5 mM for hematite and they were 54.7, 62.6, 5.5, and 0.2 mM for goethite, respectively. The disparity of anions in inducing hematite or goethite aggregation lay in the differences in interfacial interactions. NO3 − and Cl− could decrease the zeta potential and enhance aggregation mainly through increasing ionic strength and compressing electric double layers of hematite and goethite nanoparticles. F− and SO4 2− highly destabilized the suspensions of nanoparticles mainly through specific adsorption and then neutralizing the positive surface charges of nanoparticles. Specific adsorption of cations could increase positive surface charges and stabilize hematite and goethite nanoparticles. The Hamaker constants of hematite and goethite nanoparticles were calculated to be 2.87 × 10−20 and 2.29 × 10−20 J−1, respectively. The predicted CCC values based on DLVO theory were consistent well with the experimentally determined CCC values in NaNO3, NaCl, NaF, and Na2SO4 systems, which demonstrated that DLVO theory could successfully predict the aggregation kinetics even when specific adsorption of ions occurred.











Similar content being viewed by others
References
Amal R, Raper JA, Waite TD (1992) Effect of fulvic acid adsorption on the aggregation kinetics and structure of hematite particles. J Colloid Interface Sci 151:244–257
Antelo J, Avena M, Fiol S, López R, Arce F (2005) Effects of pH and ionic strength on the adsorption of phosphate and arsenate at the goethite-water interface. J Colloid Interface Sci 285:476–486
Atkinson RJ, Posner AM, Quirk JP (1967) Adsorption of potential-determining ions at the ferric oxide-aqueous electrolyte interface. J Phys Chem 71:550–558
Baalousha M (2009) Aggregation and disaggregation of iron oxide nanoparticles: influence of particle concentration, pH and natural organic matter. Sci Total Environ 407:2093–2101
Baalousha M, Lead JR (2012) Rationalizing nanomaterial sizes measured by atomic force microscopy, flow field-flow fractionation, and dynamic light scattering: sample preparation, polydispersity, and particle structure. Environ Sci Technol 46:6134–6142
Barrón V, Torrent J (1996) Surface hydroxyl configuration of various crystal faces of hematite and goethite. J Colloid Interface Sci 177:407–410
Breeuwsma A, Lyklema J (1973) Physical and chemical adsorption of ions in the electrical double layer on hematite (α-Fe2O3). J Colloid Interface Sci 43:437–448
Burrows ND, Hale CRH, Penn RL (2012) Effect of ionic strength on the kinetics of crystal growth by oriented aggregation. Cryst Growth Des 12:4787–4797
Chen KL, Elimelech M (2006) Aggregation and deposition kinetics of Fullerene (C60) nanoparticles. Langmuir 22:10994–11001
Chen KL, Elimelech M (2009) Relating colloidal stability of fullerene (C60) nanoparticles to nanoparticles charge and electrokinetic properties. Environ Sci Technol 43:7270–7276
Chen KL, Mylon SE, Elimelech M (2006) Aggregation kinetics of alginate-coated hematite nanoparticles in monovalent and divalent electrolytes. Environ Sci Technol 40:1516–1523
Darland JE, Inskeep WP (1997) Effects of pH and phosphate competition on the transport of arsenate. J Environ Qual 26:1133–1139
Dickson D, Liu G, Li C, Tachiev G, Cai Y (2012) Dispersion and stability of bare hematite nanoparticles: effects of dispersion tools, nanoparticle concentration, humic acid and ionic strength. Sci Total Environ 419:170–177
Duman O, Tunç S (2009) Electrokinetic and rheological properties of Na-bentonite in some electrolyte solutions. Microporous Mesoporous Mater 117:331–338
Farrah H, Pickering WF (1986) Interaction of dilute fluoride solutions with hydrous iron oxides. Aust J Soil Res 24:201–208
Fein JB, Boily J-F, Yee N, Gorman-Lewis D, Turner BF (2005) Potentiometric titrations of Bacillus subtilis cells to low pH and a comparison of modeling approaches. Geochim Cosmochim Acta 69:1123–1132
Forloni G (2012) Responsible nanotechnology development. J Nanopart Res 14:1–17
Gilbert B, Lu G, Kim CS (2007) Stable cluster formation in aqueous suspensions of iron oxyhydroxide nanoparticles. J Colloid Interface Sci 313:152–159
Hackley VA, Anderson MA (1989) Effects of short-range forces on the long-range structure of hydrous iron oxide aggregates. Langmuir 5:191–198
He YT, Wan J, Tokunaga T (2008) Kinetic stability of hematite nanoparticles: the effect of particle sizes. J Nanopart Res 10:321–332
Hochella MF Jr, Lower SK, Maurice PA, Penn RL, Sahai N et al (2008) Nanominerals, mineral nanoparticles, and earth systems. Science 319:1631–1635
Ju-Nam Y, Lead JR (2008) Manufactured nanoparticles: an overview of their chemistry, interactions and potential environmental implications. Sci Total Environ 400:396–414
Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TF, Handy RD et al (2008) Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ Toxicol Chem 27:1825–1851
Kosmulski M (2011) The pH-dependent surface charging and points of zero charge V. Update. J Colloid Interface Sci 353:1–15
Liang L, Morgan JJ (1990) Chemical aspects of iron oxides coagulation in water: laboratory studies and implications for natural systems. Aquat Sci 52:32–55
Lin MY, Lindsay HM, Weitz DA, Ball RC, Klein R (1989) Universality in colloid aggregation. Nature 339:360–362
Lin D, Tian X, Wu F, Xing B (2010) Fate and transport of engineered nanomaterials in the environment. J Environ Qual 39:1896–1908
Missana T, Benedicto A, Mayordomo N, Alonso U (2014) Analysis of anion adsorption effects on alumina nanoparticles stability. Appl Geochem 49:68–76
Mylon SE, Chen KL, Elimelech M (2004) Influence of natural organic matter and ionic composition on the kinetics and structure of hematite colloid aggregation: implication to iron depletion in estuaries. Langmuir 20:9000–9006
Nowack B, Bucheli TD (2007) Occurrence, behavior and effects of nanoparticles in the environment. Environ Pollut 150:5–22
Parfitt RL (1979) Anion adsorption by soils and soil materials. Adv Agron 30:1–50
Pepper SE, Hull LC, Bottenus BN, Clark SB (2006) Adsorption of lanthanum to goethite in the presence of gluconate. Radiochim Acta 94:229–237
Petosa AR, Jaisi DP, Quevedo IR, Elimelech M, Tufenkji N (2010) Aggregation and deposition of engineered nanomaterials in aquatic environments: role of physicochemical interactions. Environ Sci Technol 44:6532–6549
Qafoku NP (2010) Terrestrial nanoparticles and their controls on soil-/geo-processes and reactions. Adv Agron 107:33–91
Schudel M, Behrens SH, Holthoff H, Kretzschmar R, Borkovec M (1997) Absolute aggregation rate constants of hematite particles in aqueous suspensions: a comparison of two different surface morphologies. J Colloid Interface Sci 196:241–253
Schwertmann U, Cornell RM (2000) Iron oxides in the laboratory: preparation and characterization, 2nd edn. VCH, Weinheim
Tunç S, Duman O, Kanci B (2012) Rheological measurements of Na-bentonite and sepiolite particles in the presence of tetradecyltrimethylammonium bromide, sodium tetradecyl sulfonate and Brij 30 surfactants. Colloid Surf A 398:37–47
Wang X, Liu F, Tan W, Li W, Feng X et al (2013) Characteristics of phosphate adsorption-desorption onto ferrihydrite: comparison with well-crystalline Fe (hydr)oxides. Soil Sci 178:1–11
Waychunas GA, Kim CS, Banfield JF (2005) Nanoparticulate iron oxide minerals in soils and sediments: unique properties and contaminant scavenging mechanisms. J Nanopart Res 7:409–433
Wu Z, Luo J, Guo H, Wang X, Yang C (2001) Adsorption isotherms of lanthanum to soil constituents and effects of pH, EDTA and fulvic acid on adsorption of lanthanum onto goethite and humic acid. Chem Spec Bioavailab 13:75–81
Zhu X, Chen H, Li W, He Y, Brookes PC et al (2014) Aggregation kinetics of natural soil nanoparticles in different electrolytes. Eur J Soil Sci 65:206–217
Acknowledgments
This study was supported by National Natural Science Foundation of China (41271010 and 41230855) and Youth Innovation Promotion Association, CAS.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xu, Cy., Deng, Ky., Li, Jy. et al. Impact of environmental conditions on aggregation kinetics of hematite and goethite nanoparticles. J Nanopart Res 17, 394 (2015). https://doi.org/10.1007/s11051-015-3198-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-015-3198-8