Abstract
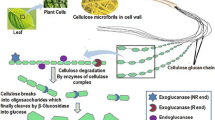
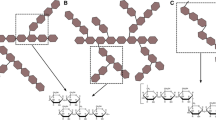
β-Glucosidases (3.2.1.21) are found in all domains of living organisms, where they play essential roles in the removal of nonreducing terminal glucosyl residues from saccharides and glycosides. β-Glucosidases function in glycolipid and exogenous glycoside metabolism in animals, defense, cell wall lignification, cell wall β-glucan turnover, phytohormone activation, and release of aromatic compounds in plants, and biomass conversion in microorganisms. These functions lead to many agricultural and industrial applications. β-Glucosidases have been classified into glycoside hydrolase (GH) families GH1, GH3, GH5, GH9, and GH30, based on their amino acid sequences, while other β-glucosidases remain to be classified. The GH1, GH5, and GH30 β-glucosidases fall in GH Clan A, which consists of proteins with (β/α)8-barrel structures. In contrast, the active site of GH3 enzymes comprises two domains, while GH9 enzymes have (α/α)6 barrel structures. The mechanism by which GH1 enzymes recognize and hydrolyze substrates with different specificities remains an area of intense study.




Similar content being viewed by others
References
Henrissat B (1991) A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J 280:309–316
Henrissat B, Davies G (1997) Structural and sequence-based classification of glycosyl hydrolases. Curr Opin Struct Biol 7:637–644
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B (2009) The Carbohydrate-Active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res 37:D233–D238
Brunner R, Wirtz W, Rose JKC, Darbill AG, Govers F, Scheel D, Nurnberger T (2002) A β-glucosidase/xylosidase from the phytopathogenic oomycete, Phytophthora infestans. Phytochemistry 59:689–696
Opassiri R, Pomthong B, Akiyama T, Nakphaichit M, Onkoksoong T, Ketudat-Cairns M, Ketudat Cairns JR (2007) A stress-induced rice β-glucosidase represents a new subfamily of glycosyl hydrolase family 5 containing a fascin-like domain. Biochem J 408:241–249
Butters TD (2007) Gaucher disease. Curr Opin Chem Biol 11:412–418
Dvir H, Harel M, McCarthy AA, Toker L, Silman I, Futerman AH, Sussman JL (2003) X-ray structure of human acid-β-glucosidase, the defective enzyme in Gaucher disease. EMBO Rep 4:704–709
Liou B, Kazimierczuk A, Zhang M, Scott CR, Hedge RS, Grabowski GA (2006) Analyses of variant acid β-glucosidases: effects of Gaucher disease mutations. J Biol Chem 281:4242–4253
Lieberman RL, Wustman BA, Huertas P, Powe AC, Pine CW, Khanna R, Schlossmacher MG, Ringe D, Petsko GA (2007) Structure of acid β-glucosidase with pharmacological chaperone provides insight into Gaucher disease. Nat Chem Biol 3:101–107
Yildiz Y, Matern H, Thompson B, Allegood JC, Warren RL, Ramirez DMO, Hammer RE, Hamra FK, Matern S, Russell DW (2006) Mutation of β-glucosidase 2 causes glycolipid storage disease and impaired male fertility. J Clin Invest 116:2985–2994
Boot RG, Verhoek M, Donker-Koopman W, Strijland A, van Marle J, Overkleeft HS, Wennekes T, Aerts JM (2007) Identification of the non-lysosomal glucosylceramidase as beta-glucosidase 2. J Biol Chem 282:1305–1312
Matern H, Boermans H, Lottspeich F, Matern S (2001) Molecular cloning and expression of human bile acid beta-glucosidase. J Biol Chem 276:37929–37933
Tribolo S, Berrin J-G, Kroon PA, Czjzek M, Juge N (2007) The structure of human cytoplasmic β-glucosidase unravels substrate aglycone specificity of a family 1 glycoside hydrolase. J Mol Biol 370:964–975
Mantei N, Villa M, Enzler T, Wacker H, Boll W, James P, Hunziker W, Semenza G (1988) Complete primary structure of human and rabbit lactase-phlorizin hydrolase: implications for biosynthesis, membrane anchoring and evolution of the enzyme. EMBO J 7:2705–2713
Arribas JC, Herrero AG, Martín-Lomas M, Cañada FJ, He S, Withers SG (2000) Differential mechanism-based labeling and unequivocal activity assignment of the two active sites of intestinal lactase/phlorizin hydrolase. Eur J Biochem 267:6996–7005
Day AJ, Canada FJ, Diaz JC, Kroon PA, Mclauchlan R, Faulds CB, Plumb GW, Morgan MR, Williamson G (2000) Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett 468:166–170
Daniels LB, Coyle PJ, Chiao YB, Glew RH, Labow RS (1981) Purification and characterization of a cytosolic broad specificity beta-glucosidase from human liver. J Biol Chem 256:13004–13013
Hayashi Y, Okino N, Kakuta Y, Shikanai T, Tani M, Narimatsu H, Ito M (2007) Klotho-related protein is a novel cytosolic neutral beta-glycosylceramidase. J Biol Chem 282:30889–30900
Day AJ, DuPont MS, Ridley S, Rhodes M, Rhodes MJ, Morgan MR, Williamson G (1998) Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver beta-glucosidase activity. FEBS Lett 436:71–75
Berrin J-G, McLauchlan WR, Needs P, Williamson G, Puigserver A, Kroon PA, Juge N (2002) Functional expression of human liver cytosolic β-glucosidase in Pichia pastoris. Insights into its role in the metabolism of dietary glucosides. Eur J Biochem 269:249–258
Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG (2005) The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science 310:490–493
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsuki T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45–51
Nabeshima Y, Imura H (2008) α-Klotho: a regulator that integrates calcium homostasis. Am J Nephrol 28:455–464
Ito S, Fujimori T, Hayashizaki Y, Nabeshima Y (2002) Identification of a novel mouse membrane-bound family 1 glycosidase-like protein, which carries an atypical active site structure. Biochim Biophys Acta 1576:341–345
Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, Nagai R, Kuro-o M, Nabeshima Y (1998) Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett 424:6–10
Zagrobelny M, Bak S, Møller BL (2008) Cyanogenesis in plants and arthropods. Phytochemistry 69:1457–1468
Marana SR, Jacobs-Lorena M, Terra WR, Ferrieira C (2001) Amino acid residues involved in substrate binding and catalysis in an insect digestive β-glycosidase. Biochim Biophys Acta 1545:41–52
Ferrieira AHP, Marana SR, Terra WR, Ferreira C (2001) Purification, molecular cloning, and properties of a β-glycosidase isolated from midgut lumen of Tenebrio molitor (Coleoptera) larvae. Insect Biochem Mol Biol 31:1065–1076
Jones AME, Bridges M, Bones AM, Cole R, Rossiter JT (2001) Purification and characterisation of a non-plant myrosinase from the cabbage aphid Brevicoryne brassicae. Insect Biochem Mol Biol 31:1–5
Malboobi MA, Lefebvre DD (1997) A phosphate-starvation inducible β-glucosidase gene (psr3.2) isolated from Arabidopsis thaliana is a member of a distinct subfamily of the BGA family. Plant Mol Biol 34:57–68
van de Ven WT, LeVesque CS, Perring TM, Walling LL (2000) Local and systemic changes in squash gene expression in response to silver winged whitefly feeding. Plant Cell 12:1409–1423
Kawasaki S, Borchert C, Deyholos M, Wang H, Brazille S, Kawai K, Galbraith D, Bohnert HJ (2001) Gene expression profiles during the initial phase of salt stress in rice. Plant Cell 13:889–905
Thorlby G, Fourier N, Warren G (2004) The SENSITIVE TO FREEZING2 gene, required for freezing tolerance in Arabidopsis thaliana, encodes a beta-glucosidase. Plant Cell 16:2192–2203
Lipka V, Dittgen J, Bednarek P, Bhat R, Wiermer M, Stein M, Landtag J, Brandt W, Rosahl S, Scheel D, Llorente F, Molina A, Parker J, Somerville S, Schulze-Lefert P (2005) Pre- and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science 310:1180–1183
Xu Z, Escamilla-Treviño LL, Zeng L, Lalgondar M, Bevan DR, Winkel BSJ, Mohamed A, Cheng C, Shih M, Poulton JE, Esen A (2004) Functional genomic analysis of Arabidopsis thaliana glycoside hydrolase family 1. Plant Mol Biol 55:343–367
Opassiri R, Pomthong B, Okoksoong T, Akiyama T, Esen A, Ketudat Cairns JR (2006) Analysis of rice glycosyl hydrolase family 1 and expression of Os4bglu12 β-glucosidase. BMC Plant Biol 6:33
Marques AR, Coutinho PM, Videira P, Fialho AM, S-Correia I (2003) Sphingomonas paucimobilis beta-glucosidase Bgl1: a member of a new bacterial subfamily in glycoside hydrolase family 1. Biochem J 370:793–804
Kuntothom T, Luang S, Harvey AJ, Fincher GB, Opassiri R, Hrmova M, Ketudat Cairns JR (2009) Rice family GH1 glycosyl hydrolases with β-d-glucosidase and β-d-mannosidase activities. Arch Biochem Biophys 491:84–95
Arthan D, Kittakoop P, Esen A, Svasti J (2006) Furostanol glycoside 26-O-β-glucosidase from the leaves of Solanum torvum. Phytochemistry 67:27–33
Niemeyer HM (1988) Hydroxamic acids (4-hydroxy-1,4-benzoxazin-3-ones), defense chemicals in the Gramineae. Phytochemistry 27:3349–3358
Poulton JE (1990) Cyanogenesis in plants. Plant Physiol 94:401–405
Morant AV, Jørgensen K, Jørgensen C, Paquette SM, Sánchéz-Perez R, Møller BL, Bak S (2008) β-Glucosidases as detonators of plant chemical defense. Phytochemistry 69:1795–1813
Sherameti I, Venus Y, Drzewiecki C, Tripathi W, Dan VM, Nitz I, Varma A, Grundler F, Oelmüller R (2008) PYK10, a β-glucosidase located in the endoplasmatic reticulum, is crucial for the beneficial interaction between Arabidopsis thanliana and the endophytic fungus Piriformospora indica. Plant J 54:428–439
Morant AV, Bjarnholt N, Kragh ME, Kjaergaard CH, Jørgensen K, Paquette SM, Piotrowski M, Imberty A, Olsen CE, Møller BL, Bak S (2008) The beta-glucosidases responsible for bioactivation of hydroxynitrile glucosides in Lotus japonicus. Plant Physiol 147:1072–1091
Suzuki H, Takahasi S, Watanabe R, Fukushima Y, Fujita N, Noguchi A, Yokoyama R, Nishitani K, Nishino T, Nakayama T (2006) An isoflavone conjugate-hydrolyzing β-glucosidase from the roots of soybean (Glycine max) seedlings. J Biol Chem 281:30251–30259
Chuankhayan P, Hua Y, Svasti J, Sakdarat S, Sullivan PA, Ketudat Cairns JR (2005) Purification of an isoflavonoid 7-O-β-apiosyl-glucoside β-glycosidase and its substrates from Dalbergia nigrescens Kurz. Phytochemistry 66:1880–1889
Chuankhayan P, Rimlumduan T, Tantanuch W, Mothong N, Kongsaeree PT, Metheenukul P, Svasti J, Jensen ON, Ketudat Cairns JR (2007) Functional and structural differences between isoflavonoid β-glycosidases from Dalbergia sp. Arch Biochem Biophys 468:205–216
Naoumkina M, Farag MA, Sumner LW, Tang Y, Liu CJ, Dixon RA (2007) Different mechanisms for phytoalexin induction by pathogen and wound signals in Medicago truncatula. Proc Natl Acad Sci USA 104:17909–17915
Esen A (1992) Purification and partial characterization of maize (Zea mays L.) β-glucosidase. Plant Physiol 98:174–182
Babcock GD, Esen A (1994) Substrate specificity of maize β-glucosidase. Plant Sci 101:31–39
Nikus J, Daniel G, Jonsson LM (2001) Subcellular localization of beta-glucosidase in rye, maize and wheat seedlings. Plant Physiol 111:466–472
Sue M, Yamazaki K, Yajima S, Nomura T, Matsukawa T, Iwamura H, Miyamoto T (2006) Molecular and structural characterization of hexameric beta-d-glucosidases in wheat and rye. Plant Physiol 141:1237–1247
Nisius A (1988) The stroma centre in Avena plastids: an aggregation of β-glucosidase responsible for the activation of oat-leaf saponins. Planta 173:474–481
Ahn YO, Shimizu B, Sakata K, Gantulga D, Zhou Z, Bevan DR, Esen A (2010) Scopulin-hydrolyzing β-glucosidases in the roots of Arabidopsis. Plant Cell Physiol 51:131–143
Hara-Nishimura I, Matsushima R (2003) A wound-inducible organelle derived from endoplasmic reticulum: a plant strategy against environmental stress? Curr Opin Plant Biol 6:538–588
Matsushima R, Kondo M, Nishimura M, Hara-Nishimura I (2003) A novel ER-derived compartment, the ER body, selectively accumulates a β-glucosidase with an ER retention signal in Arabidopsis. Plant J 33:493–502
Bednarek P, Piślewska-Bednarek M, Svatoš A, Schneider B, Doubský J, Mansurova M, Humphry M, Consonni C, Panstruga R, Sanchez-Vallet A, Molina A, Schulze-Lefert P (2009) A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 323:101–106
Nagano AJ, Matsushima R, Hara-Nishimura I (2005) Activation of an ER-body-localized β-glucosidase via a cytosolic binding partner in damaged tissues of Arabidopsis thaliana. Plant Cell Physiol 46:1140–1148
Falk A, Taipalensuu J, Ek B, Lenman M, Rask L (1995) Characterization of rapeseed myrosinase-binding protein. Planta 195:387–395
Esen A, Blanchard DJ (2000) A specific β-glucosidase-aggregating factor (BGAF) is responsible for the β-glucosidase null phenotype in maize. Plant Physiol 122:563–572
Blanchard DJ, Cicek M, Chen J, Esen A (2001) Identification of beta-glucosidase aggregating factor (BGAF) and mapping of BGAF binding regions on maize beta-glucosidase. J Biol Chem 276:11895–11901
Kittur FS, Lalgondar M, Yu HY, Bevan DR, Esen A (2007) Maize β-glucosidase-aggregating factor is a polyspecific jacalin-related chimeric lectin, and its lectin domain is responsible for β-glucosidase aggregation. J Biol Chem 282:7299–7311
Nagano AJ, Fukao Y, Fujiwara M, Nishimura M, Hara-Nishimura I (2008) Antagonistic jacalin-related lectins regulate the size of ER body-type β-glucosidase complexes in Arabidopsis thaliana. Plant Cell Physiol 49:969–980
Leah R, Kigel J, Svedsen I, Mundy J (1995) Biochemical and molecular characterization of a barley seed β-glucosidase. J Biol Chem 270:15789–15797
Hrmova M, Harvey AJ, Wang J, Shirley NJ, Jones GP, Stone BA, Hoj PB, Fincher GB (1996) Barley β-d-glucan exohydrolases with β-d-glucosidase activity. J Biol Chem 271:5277–5286
Hrmova M, MacGregor EA, Biely P, Stewart RJ, Fincher GB (1998) Substrate binding and catalytic mechanism of a barley β-d-glucosidase/(1, 4)-β-d-glucan exohydrolase. J Biol Chem 273:11134–11143
Hrmova M, Burton RA, Biely P, Lahnstein J, Fincher GB (2006) Hydrolysis of (1,4)-β-d-mannans in barley (Hordeum vulgare L.) is mediated by the concerted action of (1,4)-β-d-mannan endohydrolase and β-d-mannosidase. Biochem J 399:77–90
Akiyama T, Kaku H, Shibuya N (1998) A cell wall-bound β-glucosidase from germinated rice: purification and properties. Phytochemistry 48:49–54
Opassiri R, Ketudat Cairns JR, Akiyama T, Wara-Aswapati O, Svasti J, Esen A (2003) Characterization of a rice β-glucosidase highly expressed in flower and germinating shoot. Plant Sci 165:627–638
Opassiri R, Hua Y, Wara-Aswapati O, Akiyama T, Svasti J, Esen A, Ketudat Cairns JR (2004) β-Glucosidase, exo-β-glucanase and pyridoxine transglucosylase activities of rice BGlu1. Biochem J 379:125–131
Seshadri S, Akiyama T, Opassiri R, Kuaprasert B, Ketudat Cairns J (2009) Structural and enzymatic characterization of Os3BGlu6, a rice β-glucosidase hydrolyzing hydrophobic glycosides and (1 → 3)- and (1 → 2)-linked disaccharides. Plant Physiol 151:47–58
Hösel W, Surholt E, Borgmann E (1978) Characterization of β-glucosidase isoenzymes possibly involved in lignification from chick pea (Cicer arietinum L.) cell suspension culture. Eur J Biochem 84:487–492
Dhamawardhana DP, Ellis BE, Carlson JE (1995) A β-glucosidase from lodgepole pine xylem specific for the lignin precursor coniferin. Plant Physiol 107:331–339
Escamilla-Treviño LL, Chen W, Card ML, Shih MC, Cheng CL, Poulton JE (2006) Arabidopsis thaliana β-Glucosidases BGLU45 and BGLU46 hydrolyse monolignol glucosides. Phytochemistry 67:1651–1660
Schliemann W (1984) Hydrolysis of conjugated gibberellins by β-glucosidases from dwarf rice (Oryza sativa L. cv. Tan-ginbozu). J Plant Physiol 116:123–132
Brzobohatý B, Moore I, Kristoffersen P, Bako L, Campos N, Schell J, Palme K (1993) Release of active cytokinin by a β-glucosidase localized to the maize root meristem. Science 262:1051–1054
Dietz K-J, Sauter A, Wichert K, Messdaghi D, Hartung W (2000) Extracellular β-glucosidase activity in barley involved in the hydrolysis of ABA glucose conjugate in leaves. J Exp Bot 51:937–944
Jakubowska A, Kawalczyk S (2005) A specific enzyme hydrolyzing 6-O(4-O)-indole-3-ylacetyl-β-d-glucose in immature kernels of Zea mays. J Plant Physiol 162:207–213
Lee KH, Piao HL, Kim HY, Choi SM, Jiang F, Hartung W, Hwang I, Kwak JM, Lee IJ (2006) Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell 126:1109–1120
Stöckigt J, Zenk MH (1977) Strictosidine (isovincoside): the key intermediate in the biosynthesis of monoterpenoid indole alkaloids. J Chem Soc Chem Commun 1977:646–648
Barleben L, Panjikar S, Ruppert M, Koepke J, Stöckigt J (2007) Molecular architecture of strictosidine glucosidase: the gateway to the biosynthesis of the monoterpenoid indole alkaloid family. Plant Cell 19:2886–2897
Warzecha H, Gerasimenko I, Kutchan TM, Stöckigt J (2000) Molecular cloning and functional bacterial expression of a plant β-glucosidase specifically involved in alkaloid biosynthesis. Phytochemistry 54:657–666
Nomura T, Quesada AL, Kutchan TM (2008) The new beta-d-glucosidase in terpenoid-isoquinoline alkaloid biosynthesis in Psychotria ipecacuanha. J Biol Chem 283:34650–34659
Reuveni M, Sagi Z, Evnor D, Hetzroni A (1999) β-Glucosidase activity is involved in scent production in Narcissus flowers. Plant Sci 147:19–24
Mattiacci L, Dicke M, Posthumus MA (1995) Beta-glucosidase: an elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc Natl Acad Sci USA 92:2036–2040
Gilbert HJ, Stålbrand H, Brumer H (2008) How the walls come tumbling down: recent structural biochemistry of plant polysaccharide degradation. Curr Opin Plant Biol 11:338–348
Doi RH, Kosugi A (2004) Cellulosomes: plant-cell-wall-degrading enzyme complexes. Nat Rev Microbiol 2:541–551
Carvalho AL, Dias FM, Nagy T, Prates JA, Proctor MR, Smith N, Bayer EA, Davies GJ, Ferreira LM, Romaño MJ, Fontes CM, Gilbert HJ (2007) Evidence for a dual binding mode of dockerin modules to cohesins. Proc Natl Acad Sci USA 2007(104):3089–3094
Lymar ES, Li B, Renganathan V (1995) Purification and characterization of a cellulose-binding β-glucosidase from cellulose-degrading cultures of Phanerochaete chrysosporium. Appl Environ Microbiol 61:2976–2980
Igarashi K, Tani T, Kawal R, Samejima M (2003) Family 3 β-glucosidase from cellulose-degrading culture of the white-rot fungus Phanerochaete chrysosporium. J Biosci Bioeng 95:572–576
Tsukada T, Igarashi K, Yoshida M, Samejima M (2006) Molecular cloning and characterization of two intracellular β-glucosidases belonging to glycoside hydrolase family 1 from the basidiomycete Phanerochaete chrysosporium. Appl Microbiol Biotechnol 73:807–814
Günata Z (2003) Flavor enhancement in fruit juices and derived beverages by exogenous glycosidases and consequences of the use of enzyme preparations. In: Whitaker JR, Voragen AGJ, Wong DWS (eds) Handbook of food enzymology. Marcel Dekker Inc., New York, pp 303–330
Esen A (2003) β-Glucosidases. In: Whitaker JR, Voragen AGJ, Wong DWS (eds) Handbook of food enzymology. Marcel Dekker Inc., New York, pp 791–804
Yasumoto K, Tsuji H, Iwami K, Mitsuda H (1977) Isolation from rice bran of a bound form of vitamin B6 and its identification as 5′-O-β-d-glucopyranosyl-pyridoxine. Agric Biol Chem 41:1061–1067
Chuankhayan P, Rimlumduan T, Svasti J, Ketudat Cairns JR (2007) Hydrolysis of soybean isoflavonoid glycosides by Dalbergia β-glucosidases. J Agric Food Chem 55:2407–2412
Ismail B, Hayes K (2005) β-Glycosidase activity toward different glycosidic forms of isoflavones. J Agric Food Chem 53:4918–4924
Higdon JV, Delage B, Williams DE, Dashwood RH (2007) Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res 55:224–236
Sánchez-Pérez R, Jørgensen K, Olsen CE, Dicenta F, Møller BL (2008) Bitterness in almonds. Plant Physiol 146:1040–1052
Crout DH, Vic G (1998) Glycosidases and glycosynthetases in glycoside and oligosaccharide synthesis. Curr Opin Chem Biol 2:98–111
Henrissat B, Callebaut I, Fabrega S, Lehn P, Mornon JP, Davies G (1995) Conserved catalytic machinery and the prediction of a common fold for several families of glycosyl hydrolases. Proc Natl Acad Sci USA 92:7090–7094
Jenkins J, Lo Leggio L, Harris G, Pickersgill R (1995) Beta-glucosidase, beta-galactosidase, family A cellulases, family F xylanases and two barley glycanases form a superfamily of enzymes with 8-fold beta/alpha architecture and with two conserved glutamates near the carboxy-terminal ends of beta-strands four and seven. FEBS Lett 362:281–285
Sanz-Aparicio J, Hermoso JA, Martinez-Ripoll M, Lequerica JL, Polaina J (1998) Crystal structure of beta-glucosidase A from Bacillus polymyxa: insights into the catalytic activity in family 1 glycosyl hydrolases. J Mol Biol 275:491–502
Varghese JN, Hrmova M, Fincher GB (1999) Three-dimensional structure of a barley β-d-glucan exohydrolase; a family 3 glycosyl hydrolase. Structure 7:179–190
Hrmova M, Varghese JN, De Gori R, Smith BJ, Driguez H, Fincher GB (2001) Catalytic mechanisms and reaction intermediates along the hydrolytic pathway of a plant beta-d-glucan glucohydrolase. Structure 9:1005–1016
Park JK, Wang L-X, Patel HV, Roseman S (2002) Molecular cloning and characterization of a unique β-glucosidase from Vibrio cholorae. J Biol Chem 277:29555–29560
Qi M, Jun H-S, Forsbert CW (2008) Cel9D, an atypical 1, 4-β-d-glucan glucohydrolase from Fibrobacter succinogenes: characteristics, catalytic residues, and synergistic interactions with other cellulases. J Bacteriol 109:1976–1984
Rye CS, Withers SG (2000) Glycosidase mechanisms. Curr Opin Chem Biol 4:573–580
Davies GJ, Ducros VM-A, Varrot A, Zechel DL (2003) Mapping the conformational itinerary of β-glucosidases by X-ray crystallography. Biochem Soc Trans 31:523–527
Czjzek M, Cicek M, Zamboni V, Bevan DR, Henrissat B, Esen A (2000) The mechanism of substrate (aglycone) specificity in β-glucosidases is revealed by crystal structures of mutant maize β-glucosidase-DIMBOA, -DIMBOAGlc, and dhurrin complexes. Proc Natl Acad Sci USA 97:13555–13560
Czjzek M, Cicek M, Zamboni V, Burmeister WP, Bevan DR, Henrissat B, Esen A (2001) Crystal structure of a monocotyledon (maize ZMGlu1) β-glucosidase and a model of its complex with p-nitrophenyl β-d-thioglucoside. Biochem J 354:37–46
Verdoucq L, Morinière J, Bevan DR, Esen A, Vasella A, Henrissat B, Czjzek M (2004) Structural determinants of substrate specificity in family 1 β-glucosidases: novel insights from the crystal structure of sorghum dhurrinase-1, a plant β-glucosidase with strict specificity, in complex with its natural substrate. J Biol Chem 279:31796–31803
Chuenchor W, Pengthaisong S, Robinson RC, Yuvaniyama J, Oonanant W, Bevan DR, Esen A, Chen C-J, Opassiri R, Svasti J, Ketudat Cairns JR (2008) Structural insights into rice BGlu1 β-glucosidase oligosaccharide hydrolysis and transglycosylation. J Mol Biol 377:1200–1215
Zechel DL, Boraston AB, Gloster TM, Boraston CM, Macdonald JM, Tilbrook DMG, Stick RV, Davies GJ (2003) Iminosugar glycosidase inhibitors: structural and thermodynamic dissection of the binding of isofagomine and 1-deoxynojirimycin to β-glucosidases. J Am Chem Soc 125:14313–14323
Vincent F, Gloster TM, Macdonald J, Morland C, Stick RV, Dias FM, Prates JA, Fontes CM, Gilbert HJ, Davies GJ (2004) Common inhibition of both beta-glucosidases and beta-mannosidases by isofagomine lactam reflects different conformational itineraries for pyranoside hydrolysis. Chembiochem 5:1596–1599
Gloster TM, Macdonald JM, Tarling CA, Stick RV, Withers SG, Davies GJ (2004) Structural, thermodynamic, and kinetic analyses of tetrahydroozazine-derived inhibitors bound to β-glucosidases. J Biol Chem 279:29236–49242
Gloster TM, Madsen R, Davies GJ (2006) Dissection of conformationally restricted inhibitors binding to a beta-glucosidase. Chembiochem 7:738–742
Gloster TM, Roberts S, Perugino G, Rossi M, Moracci M, Panday N, Terinek M, Vasella A, Davies GJ (2006) Structural, kinetic, and thermodynamic analysis of glucoimidazole-derived glycosidase inhibitors. Biochemistry 45:11879–11884
Gloster TM, Meloncelli P, Stick RV, Zechel D, Vasella A, Davies GJ (2007) Glycosidase inhibition: an assessment of the binding of 18 putative transition-state mimics. J Am Chem Soc 129:2345–2354
Withers SG, Street IP, Bird P, Dolphin DH (1987) 2-Deoxy-2-fluoroglucosides: a novel class of mechanism based inhibitors. J Am Chem Soc 109:7530–7531
Withers SG, Warren RAJ, Street IP, Rupitz K, Kempton JB, Aebersold R (1990) Unequivocal demonstration of the involvement of a glutamate residue as a nucleophile in the mechanism of a retaining glycosidase. J Am Chem Soc 112:5887–5889
Burmeister WP, Cottaz S, Driguez H, Iori R, Palmieri S, Henrissat B (1997) The crystal structures of Sinapis alba myrosinase and a covalent glycosyl-enzyme intermediate provide insights into the substrate recognition and active-site machinery of an S-glycosidase. Structure 5:663–675
Noguchi J, Hayashi Y, Baba Y, Okino N, Kimura M, Ito M, Kakuta Y (2008) Crystal structure of the covalent intermediate of human cytosolic beta-glucosidase. Biochem Biophys Res Commun 374:549–552
Keresztessy Z, Kiss L, Hughes MA (1994) Investigation of the active site of the cyanogenic beta-d-glucosidase (linamarase) from Manihot esculenta Crantz (cassava). II. Identification of Glu-198 as an active site carboxylate group with acid catalytic function. Arch Biochem Biophys 315:323–330
Wang Q, Trimbur D, Graham R, Warren RA, Withers SG (1995) Identification of the acid/base catalyst in Agrobacterium faecalis beta-glucosidase by kinetic analysis of mutants. Biochemistry 34:14554–14562
Wang Q, Graham RW, Trimbur D, Warren RAJ, Withers SG (1994) Changing enzymic reaction mechanisms by mutagenesis: conversion of a retaining glucosidase to an inverting enzyme. J Am Chem Soc 116:11594–11595
Mackenzie LF, Wang Q, Warren RAJ, Withers SG (1998) Glycosynthases: mutant glycosidases for oligosaccharide synthesis. J Am Chem Soc 120:5583–5584
Ly HD, Withers SG (1999) Mutagenesis of glycosidases. Annu Rev Biochem 68:487–522
Marana SR (2006) Molecular basis of substrate specificity in family 1 glycoside hydrolases. IUBMB Life 58:63–73
Verdoucq L, Czjzek M, Moriniere J, Bevan DR, Esen A (2003) Mutational and structural analysis of aglycone specificity in maize and sorghum β-glucosidases. J Biol Chem 278:25055–25062
Berrin J-G, Czjzek M, Kroon PA, McLauchlan WR, Puigserver A, Williamson G, Juge N (2003) Substrate (aglycone) specificity of human cytosolic β-glucosidase. Biochem J 373:41–48
Cicek M, Blanchard D, Bevan DR, Esen A (2000) The aglycone specificity-determining sites are different in 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one (DIMBOA)-glucosidase (maize beta-glucosidase) and dhurrinase (sorghum beta-glucosidase). J Biol Chem 275:20002–20011
Zouhar J, Vévodová J, Marek J, Damborský J, Su X-D, Bryzobohatý B (2001) Insights into the functional architecture of the catalytic center of a maize β-glucosidase Zm-p60.1. Plant Physiol 127:973–985
Srisomsap C, Svasti J, Surarit R, Champattanachai V, Sawangareetrakul P, Boonpuan K, Subhasitanont P, Chokchaichamnankit D (1996) Isolation and characterization of an enzyme with beta-glucosidase and beta-fucosidase activities from Dalbergia cochinchinensis Pierre. J Biochem 119:585–590
Zollner H (1989) Handbook of enzyme inhibitors. VCH, Weinheim, pp 94–95
Burmeister WP, Cottaz S, Rollin P, Vasella A, Henrissat B (2000) High resolution X-ray crystallography shows that ascorbate is a cofactor for myrosinase and substitutes for the function of the catalytic base. J Biol Chem 275:39385–39393
Kengen SW, Luesink EJ, Stams AJ, Zehnder AJ (1993) Purification and characterization of an extremely thermostable beta-glucosidase from the hyperthermophilic archaeon Pyrococcus furiosus. Eur J Biochem 213:305–312
Nam KH, Kim S-J, Kim M-Y, Kim JH, Yeo Y-S, Lee C-M, Jun H-K, Hwang KY (2008) Crystal structure of engineered beta-glucosidase from soil metagenome. Proteins 73:788–793
Chi YI, Martinez-Cruz LA, Jancarik J, Swanson RV, Robertson DE, Kim SH (1999) Crystal structure of the beta-glycosidase from the hyperthermophile Thermosphaera aggregans: insights into its activity and thermostability. FEBS Lett 445:375–383
Acknowledgments
Rodjana Opassiri and three anonymous reviewers are thanked for useful comments on the manuscript. JRKC was supported by Suranaree University of Technology and the Thailand Research Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ketudat Cairns, J.R., Esen, A. β-Glucosidases. Cell. Mol. Life Sci. 67, 3389–3405 (2010). https://doi.org/10.1007/s00018-010-0399-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-010-0399-2