Abstract
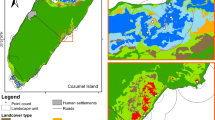
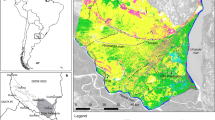
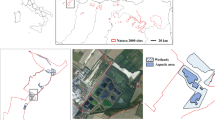
Management of wetland connectivity is important for biodiversity conservation. In the modern agricultural landscape, the natural connections between floodplain wetlands have been greatly altered. Agricultural ditches and channelized streams are widely distributed in floodplains, which may contribute to the maintenance of wetland connectivity and biodiversity. To determine how these watercourse networks affect wetland biodiversity, we examined the relationship between the species richness of aquatic animals and wetland connectivity, with a special focus on species mobility. From July to August 2011, fish and aquatic insects were collected from 24 wetlands in northern Japan. To determine the degree of wetland connectivity, we assessed the relative importance of individual wetlands in maintaining the entire wetland network using two connectivity indices: hydrologic connectivity via watercourses and spatial connectivity defined as Euclidian distances between wetlands using graph theory. We found that only high mobility groups of both taxa could enhance species richness in either a hydrologic (fish) or spatial (insect) wetland network. The species richness of insects with high-flying ability was found to increase as spatial connectivity increased. Furthermore, the species richness of fish with high-swimming ability was positively influenced by hydrologic connectivity, most likely because highly mobile species were able to reach suitable habitats and migrate from source populations in a wetland network owing to their good mobility. Our findings indicate that hydrologic network is important for maintaining biodiversity as well as spatial connectivity. It is important to focus conservation efforts on key wetlands with high hydrologic and spatial connectivity in future wetland management.





Similar content being viewed by others
References
Aarts BGW, Van Den Brink FWB, Nienhuis PH (2003) Habitat loss as the main cause of the slow recovery of fish faunas of regulated large rivers in Europe: the transversal floodplain gradient. River Res Appl 20:3–23
Ahn YS, Nakamura F, Kizuka T, Nakamura Y (2009) Elevated sedimentation in lake records linked to agricultural activities in the Ishikari River floodplain, northern Japan. Earth Surf Proc Land 34:1650–1660
Akasaka T, Akasaka M, Yanagawa H (2010) Relative importance of the environmental factors at site and landscape scales for bats along the riparian zone. Landsc Ecol Eng 6:247–255
Armitage PD, Szoszkiewicz K, Blackburn JH, Nesbitt I (2003) Ditch communities: a major contributor to floodplain biodiversity. Aquat Conserv Mar Freshw Ecosyst 13:165–185
Baber MJ, Childers DL, Babbitt KJ, Anderson DH (2002) Controls on fish distribution and abundance in temporary wetlands. Can J Fish Aquat Sci 59:1441–1450
Beier P, Noss RF (1998) Do habitat corridors provide connectivity? Conserv Biol 12:1241–1252
Bouvier LD, Cottenie K, Doka SE (2009) Aquatic connectivity and fish metacommunities in wetlands of the lower Great Lakes. Can J Fish Aquat Sci 66:933–948
Burnham KP, Anderson DR (2002) Model selection and inference: a practical information-theoretic approach, 2nd. Springer, New York
Castro-Santos T (2005) Optimal swim speeds for traversing velocity barriers: an analysis of volitional high-speed swimming behavior of migratory fishes. J Exp Biol 208:421–432
Čížková H, Květ J, Comín FA, Laiho R, Pokorný J, Pithart D (2013) Actual state of European wetlands and their possible future in the context of global climate change. Aquat Sci 75:3–26
Colwell RK (2013) EstimateS: statistical estimation of species richness and shared species for samples, Version 9.1.0. http://viceroy.eeb.uconn.edu/EstimateS/index.html. Accessed Nov 2013
Colwell RK, Chao A, Gotelli NJ, Lin SY, Mao CX, Chazdon RL, Longino JT (2012) Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J Plant Ecol 5:3–21
Economo EP, Keitt TH (2010) Network isolation and local diversity in neutral metacommunities. Oikos 119:1355–1363
Erős T, Olden JD, Schick RS, Schmera D, Fortin MJ (2012) Characterizing connectivity relationships in freshwaters using patch-based graphs. Landsc Ecol 27:303–317
Ewers RM, Didham RK (2006) Confounding factors in the detection of species responses to habitat fragmentation. Biol Rev 81:117–142
Fagan WF (2002) Connectivity, fragmentation, and extinction risk in dendritic metapopulations. Ecology 83:3243–3249
Fahrig L, Merriam G (1994) Conservation of fragmented populations. Conserv Biol 8:50–59
Fensham RJ, Price RJ (2004) Ranking spring wetlands in the Great Artesian Basin of Australia using endemicity and isolation of plant species. Biolo Conserv 119:41–50
Fortuna MA, Gómez-Rodríguez C, Bascompte J (2006) Spatial network structure and amphibian persistence in stochastic environments. Proc Biol Sci 273:1429–1434
Galat DL, Fredrickson LH, Humburg DD, Bataille KJ, Bodie JR, Dohrenwend J, Gelwicks GT, Havel JE, Helmers DL, Hooker JB (1998) Flooding to restore connectivity of regulated, large-river wetlands. Bioscience 48:721–733
Ganio LM, Torgersen CE, Gresswell RE (2005) A geostatistical approach for describing spatial pattern in stream networks. Front Ecol Environ 3:138–144
Gibbs JP (1993) Importance of small wetlands for the persistence of local populations of wetland-associated animals. Wetlands 13:25–31
Gilbert F, Gonzalez A, Evans-Freke I (1998) Corridors maintain species richness in the fragmented landscapes of a microecosystem. Proc Biol Sci 265:577–582
Godfray HCJ, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, Pretty J, Robinson S, Thomas SM, Toulmin C (2010) Food security: the challenge of feeding 9 billion people. Science 327:812–818
Harrison SSC, Bradley DC, Harris IT (2005) Uncoupling strong predator-prey interactions in streams: the role of marginal macrophytes. Oikos 108:433–448
Heino J (2013) Does dispersal ability affect the relative importance of environmental control and spatial structuring of littoral macroinvertebrate communities? Oecologia 171:971–980
Hepp LU, Santos S (2009) Benthic communities of streams related to different land uses in a hydrographic basin in southern Brazil. Environ Monit Assess 157:305–318
Jackson DA, Peres-Neto PR, Olden JD (2001) What controls who is where in freshwater fish communities the roles of biotic, abiotic, and spatial factors. Can J Fish Aquat Sci 58:157–170
Johnston CA (1994) Cumulative impacts to wetlands. Wetlands 14:49–55
Katayama N, Saitoh D, Amano T, Miyashita T (2011) Effects of modern drainage systems on the spatial distribution of loach in rice ecosystems. Aquat Conserv Mar Freshw Ecosyst 21:146–154
Kawai T, Tanida K (2005) Aquatic insects of Japan: Manual with keys and illustrations. Toukai university press, Kanagawa
Kawanabe Y, Mizuno N (1998) Freshwater fish of Japan. Yama-to-keikokusya, Tokyo
Keddy PA (1992) Assembly and response rules: two goals for predictive community ecology. J Veg Sci 3:157–164
Kohn DD, Walsh DM (1994) Plant species richness-the effect of island size and habitat diversity. J Ecol 82:367–377
Lucas MC, Baras E, Thom TJ, Duncan A, Slavlk O (2001) Migration of freshwater fishes. Blackwell Science, Oxford
Maltchik L, Lanés LEK, Stenert C, Medeiros ESF (2010) Species-area relationship and environmental predictors of fish communities in coastal freshwater wetlands of southern Brazil. Environ Biol Fish 88:25–35
Matsuzaki SS, Terui A, Kodama K, Tada M, Yoshida T, Washitani I (2011) Influence of connectivity, habitat quality and invasive species on egg and larval distributions and local abundance of crucian carp in Japanese agricultural landscapes. Biolo Conserv 144:2081–2087
Merritt RW, Cummins KW (1996) Aquatic insects of North America. Kendall Hunt, Dubuque
Merritt RW, Wallace JR, Higgins MJ, Alexander MK, Berg MB, Morgan WT, Cummins KW, Vandeneeden B (1996) Procedures for the functional analysis of invertebrate communities of the Kissimmee River-floodplain ecosystem. Fla Sci 59:216–274
Minor ES, Urban DL (2008) A graph-theory framework for evaluating landscape connectivity and conservation planning. Conserv Biol 22:297–307
Mitsuo Y, Ohira M, Tsunoda H, Yuma M (2013) Movement patterns of small benthic fish in lowland headwater streams. Freshw Biol 58:2345–2354
Miyazono S, Aycock JN, Miranda LE, Tietjen TE (2010) Assemblage patterns of fish functional groups relative to habitat connectivity and conditions in floodplain lakes. Ecol Freshw Fish 19:578–585
Moser M, Prentice C, Frazier S (1996) A global overview of wetland loss and degradation. http://www.ramsar.org/cda/en/ramsar-news-archives-2002–a-global-overview-of/main/ramsar/1-26-45-87%5E16905_4000_0__. Accessed April 2013
Nürnberger B (1996) Local dynamics and dispersal in a structured population of the whirligig beetle Deneutus assimilis. Oecologia 106:325–336
Obihiro Development and Construction Department. http://www.ob.hkd.mlit.go.jp/. Accessed April 2013
Öckinger E, Franzen M, Rundlof M, Smith HG (2009) Mobility-dependent effects on species richness in fragmented landscapes. Basic Appl Ecol 10:573–578
Öckinger E, Schweiger O, Crist TO, Debinski DM, Krauss J, Kuussaari M, Petersen JD, Pöyry J, Settele J, Summerville KS (2010) Life-history traits predict species responses to habitat area and isolation: a cross-continental synthesis. Ecol Lett 13:969–979
Oertli B, Indermuehle N, Angélibert S, Hinden H, Stoll A (2008) Macroinvertebrate assemblages in 25 high alpine ponds of the Swiss National Park (Cirque of Macun) and relation to environmental variables. Hydrobiologia 597:29–41
Pascual-Hortal L, Saura S (2006) Comparison and development of new graph-based landscape connectivity indices: towards the priorization of habitat patches and corridors for conservation. Landsc Ecol 21:959–967
Pereira M, Segurado P, Neves N (2011) Using spatial network structure in landscape management and planning: a case study with pond turtles. Landsc Urban Plan 100:67–76
Poff NLR, Olden JD, Vieira NKM, Finn DS, Simmons MP, Kondratieff BC (2006) Functional trait niches of North American lotic insects: traits-based ecological applications in light of phylogenetic relationships. J N Am Benthol Soc 25:730–755
Power ME, Dudley TL, Cooper SD (1989) Grazing catfish, fishing birds, and attached algae in a Panamanian stream. Environ Biol Fish 26:285–294
Power G, Brown RS, Imhof JG (1999) Groundwater and fish- insights from northern North America. Hydrol Processes 13:401–422
Purse BV, Hopkins GW, Day KJ, Thompson DJ (2003) Dispersal characteristics and management of a rare damselfly. J Appl Ecol 40:716–728
R Development Core Team (2008) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Riberio R, Carretero MA, Sillero N, Alarcos G, Ortiz-Santaliestra M, Lizana M, Llorente GA (2011) The pond network: can structural connectivity reflect on (amphibian) biodiversity patterns? Landsc Ecol 26:673–682
Saura S, Torne J (2009) Conefor Sensinode 2.2: a software package for quantifying the importance of habitat patches for landscape connectivity. Environ Model Softw 24:135–139
Scheffer M, van Nes EH (2007) Shallow lakes theory revisited: various alternative regimes driven by climate, nutrients, depth and lake size. Hydrobiologia 584:455–466
Sugimura M, Ishida S, Kojima K, Ishida S, Aoki N (1999) Dragonflies of the Japanese archipelago in color. Hokkaido university press, Hokkaido
Taniguchi H, Nakano S, Tokeshi M (2003) Influences of habitat complexity on the diversity and abundance of epiphytic invertebrates on plants. Freshw Biol 48:718–728
Tilman D (1999) Global environmental impacts of agricultural expansion: the need for sustainable and efficient practices. P Nat Acad Sci USA 96:5995–6000
Tiner RW (2003) Geographically isolated wetlands of the United States. Wetlands 23:494–516
Tockner K, Stanford JA (2002) Riverine flood plains: present state and future trends. Environ Conserv 29:308–330
Van de Meutter F, Stoks R, De Meester L (2006) Lotic dispersal of lentic macroinvertebrates. Ecography 29:223–230
Verhoeven JTA, Soons MB, Janssen R, Omtzigt N (2008) An operational landscape unit approach for identifying key landscape connections in wetland restoration. J Appl Ecol 45:1496–1503
Vieira NMK, Poff NL, Carlisle DM, Moulton SR, Koski ML, Kondratieff BC (2006) A database of lotic invertebrate traits for North America. US Geological Survey Reston, Virginia
Williams P, Whitfield M, Biggs J, Bray S, Fox G, Nicolet P, Sear D (2003) Comparative biodiversity of rivers, streams, ditches and ponds in an agricultural landscape in Southern England. Biol Conservat 115:329–341
Williamson CE, Saros JE, Vincent WF, Smol JP (2009) Lakes and reservoirs as sentinels, integrators, and regulators of climate change. Limnol Oceanogr 54:2273–2282
Zedler JB, Kercher S (2005) Wetland resources: status, trends, ecosystem services, and restorability. Annu Rev Environ Resour 30:39–74
Acknowledgments
We are grateful to Yuichi Yamaura and Masanao Sueyoshi of Hokkaido University for their conceptual and technical help. Critical and helpful comments from reviewers and an editor greatly improved the manuscript. We also thank the members of the Forest Ecosystem Management Laboratory of Hokkaido University for their help in conducting the fieldwork and for productive comments regarding the study. We thank the Ikeda, Toyokoro, and Urahoro town offices for their assistance in selecting the study sites. This study was supported by Grants in Aid for Scientific Research (No. 23248021) from the Ministry of Education, Science, and Culture, Japan; a Grant of the Environment Research and Technology Development Fund (S9 and 4D-1201) from the Ministry of the Environment of Japan; and the research fund for the Tokachi River provided by the Ministry of Land, Infrastructure, Transport, and Tourism of Japan.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1 Individual-based rarefaction curves for estimated fish species richness in each wetland. Numbers beside each curve indicate the wetland ID. Numbers in parentheses denote the abundance of fish captured in each wetland.
Supplementary Fig. 2 Sample-based rarefaction curves for estimated insect species richness in each wetland. Numbers beside each curve indicate the wetland ID. Numbers in parentheses denote the number of sampling points in each wetland.
Rights and permissions
About this article
Cite this article
Ishiyama, N., Akasaka, T. & Nakamura, F. Mobility-dependent response of aquatic animal species richness to a wetland network in an agricultural landscape. Aquat Sci 76, 437–449 (2014). https://doi.org/10.1007/s00027-014-0345-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00027-014-0345-8