Abstract
A fundamental property of most animals is the ability to see whether an object is approaching on a direct collision course and, if so, when it will collide. Using high-density electroencephalography in 5- to 11-month-old infants and a looming stimulus approaching under three different accelerations, we investigated how the young human nervous system extracts and processes information for impending collision. Here, we show that infants’ looming related brain activity is characterised by theta oscillations. Source analyses reveal clear localised activity in the visual cortex. Analysing the temporal dynamics of the source waveform, we provide evidence that the temporal structure of different looming stimuli is sustained during processing in the more mature infant brain, providing infants with increasingly veridical time-to-collision information about looming danger as they grow older and become more mobile.




Similar content being viewed by others
References
Assmus A, Marshall JC, Ritzl A, Noth J, Zilles K, Fink GR (2003) Left inferior parietal cortex integrates time and space during collision judgments. NeuroImage 20:S82–S88
Coch D, Skendzel W, Grossi G, Neville H (2005) Motion processing in school-age children and adults, an ERP study. Dev Sci 8:372–386
Coull JT, Vidal F, Goulon C, Nazarian B, Craig C (2008) Using time-to-contact information to assess potential collision modulates both visual and temporal prediction networks. Frontiers in Human Neurosci 2:1–12
De Haan M, Thomas M (2002) Application of ERP and fMRI techniques to developmental science. Dev Sci 5:335–343
Di Russo F, Pitzalis S, Spitoni G, Aprile T, Patria F, Spinelli D, Hillyard SA (2005) Identification of the neural sources of the pattern-reversal VEP. NeuroImage 24:874–886
Dougherty RF, Koch VM, Brewer AA, Fischer B, Modersitzki J, Wandell BA (2003) Visual field representations and locations of visual areas V1/2/3 in human visual cortex. J Vision 3:586–598
Field DT, Wann JP (2005) Perceiving time to collision activates the sensorimotor cortex. Curr Biol 15:453–458
Gilmore RO, Baker TJ, Grobman KH (2004) Stability in young infants’ discrimination of optic flow. Dev Psychol 40:259–270
Grieve PG, Emerson RG, Fifer WP, Isler JR, Stark RI (2003) Spatial correlation of the infant and adult electroencephalogram. Clin Neurophysiol 114:1594–1608
Hatsopoulos N, Gabbiani F, Laurent G (1995) Elementary computation of object approach by a wide-field visual neuron. Science 270:1000–1003
Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M (2004) BESA source coherence: a new method to study cortical oscillatory coupling. Brain Topogr 16:233–238
Hollants-Gilhuijs MAM, De Munck JC, Kubova Z, Van Royen E, Spekreijse H (2000) The development of hemispheric asymmetry in human motion VEPs. Vis Res 40:1–11
Holliday IE, Meese TS (2005) Neuromagnetic evoked responses to complex motions are greatest for expansion. Int J Psychophysiol 55:145–157
Johnson MH (2000) Functional brain development in infants: elements of an interactive specialization framework. Child Dev 71:75–81
Kahana MJ, Seelig D, Madsen JM (2001) Theta returns. Curr Opin Neurobiol 11:739–744
Kayed NS, Van der Meer ALH (2007) Infants’ timing strategies to optical collisions: a longitudinal study. Infant Behav Dev 30:50–59
Kayed NS, Van der Meer ALH (2009) A longitudinal study of prospective control in catching by full-term and preterm infants. Exp Brain Res 194:245–258
Langrova J, Kuba M, Kremlacek J, Vit F (2006) Motion onset VEPs reflect long maturation and early ageing of visual motion-processing system. Vis Res 46:536–544
Lee DN (1998) Guiding movement by coupling taus. Ecol Psychol 10:221–250
Lee DN, Georgopoulos AP, Clark MJO, Craig CM, Port NL (2001) Guiding contact by coupling the taus of gaps. Exp Brain Res 139:151–159
Maier JX, Ghazanfar AA (2007) Looming biases in monkey auditory cortex. J Neurosci 27:4093–4100
Martinoya CJ, Delius D (1990) Perception of rotating spiral patterns by pigeons. Biol Cybern 63:127–134
Mehta MR, Lee AK, Wilson MA (2002) Role of experience and oscillations in transforming a rate code into a temporal code. Nature 417:741–746
Morrone MC, Tosetti M, Montanara D, Fiorentini A, Cioni G, Burr DC (2000) A cortical area that responds specifically to optic flow, revealed by fMRI. Nature Neurosci 3:1322–1328
O’Keefe J, Recce ML (1993) Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 3:317–330
Orekhova EV, Stroganova TA, Posikera IN (1999) Theta synchronization during sustained anticipatory attention in infants over the second half of the first year of life. Int J Psychophysiol 32:151–172
Pfurtscheller G, Lopes da Silva FH (1999) Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 110:1842–1857
Ptito M, Kupers R, Faubert J, Gjedde A (2001) Cortical representation of inward and outward radial motion in man. NeuroImage 14:1409–1415
Rind FC, Simmons PJ (1997) Signaling of object approach by the DCMD neuron of the locust. J Neurophysiol 77:1029–1033
Scherg M (1990) Fundamentals of dipole source potential analysis. In: Grandori F, Hoke M, Romani GL (eds) Auditory evoked magnetic fields and electric potentials: advances in audiology. Karger, Basel, pp 40–69
Scherg M (2002) Advanced tools for digital EEG review: virtual source montages, whole-head mapping, correlation, and phase analysis. J Clin Neurophysiol 19:91–112
Schiff W (1965) Perception of impending collision: a study of visually directed avoidant behavior. Psychol Monogr 79:1–26
Schiff W, Caviness JA, Gibson JJ (1962) Persistent fear response in rhesus monkeys to the optical stimulus of “looming”. Science 136:982–983
Shirai N, Yamaguchi MK (2004) Asymmetry in the perception of motion-in-depth. Vis Res 44:1003–1011
Sun H, Frost BJ (1998) Computation of different optical variables of looming objects in pigeon nucleus rotundus neurons. Nature Neurosci 1:296–303
Tallon-Baudry C, Bertrand O, Perronnet F, Pernier J (1998) Induced gamma-band activity during the delay of a visual short-term memory task in humans. J Neuroscience 18:4244–4254
Tucker DM (1993) Spatial sampling of head electric fields: the Geodesic sensor net. Electroencephalogr Clin Neurophysiol 87:154–163
Vinogradova OS (1995) Expression, control, and probable functional significance of the neuronal theta-rhythm. Prog Neurobiol 45:523–583
Webb SJ, Long JD, Nelson CA (2005) A longitudinal investigation of visual event-related potentials in the first year of life. Dev Sci 8:605–616
Acknowledgements
We are grateful to all the infants and their parents for taking part in this study. We also thank D. N. Lee for discussion, G. -J. Pepping for providing us with the tau analysis software, S. Houweling and J. F. Léger for programming the looming stimuli and M. Holth for testing assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary 1
(a) Raw EEG data of a single 2s (fast) looming trial displayed using standard 10–20 sites. Note increased activity at sites O1, Oz and O2 as a direct response to the loom, with vertical yellow line marking peak activity. The inserted 3D mapping window visualises a buildup and decline over time of EEG voltage in the visual cortex (0 ms = peak activity at vertical yellow line) (see Supplementary Video 3). (b) Source analysis of the same trial, using a predefined surrogate source model of the visual areas (Scherg 2002), including O1, Oz and O2. Dipoles at these sites were fitted −200:200 ms around peak VEP activity as indicated by yellow line in (a), providing source waveforms (SWF) of the modelled brain regions as a direct measure of their activities on a trial-by-trial basis (centre lower panel). Two dipoles, VCrL (blue curve) and VCrR (red curve), showed consistent symmetrical synchronised activity in response to our looming stimulus. Top right panel shows the relative contribution (position and direction) of each dipole to the model. Bottom right panel shows a multiple-source beamformer image of evoked brain responses to the looming stimulus, revealing involvement of primary visual areas in the occipital cortex and of the right-hemispheric hMT + area (see Supplementary Video 2). (c) Grand average motion VEPs of infants’ responses to slow (in blue), medium (in red) and fast (in black) looms across age groups. The sites shown are predominantly active during the visual processing of the looms. Red dots in model head (nose up) indicate scalp localisation of the sites. (PDF 2.01 MB)
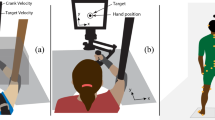
The looming stimulus. This movie shows an 8-month-old infant ready for testing and a diagram of our experimental setup (see also Fig. 1). The infant is watching the looming stimuli approaching under three different accelerations. The blue dot moving in the middle of the looming circle represents the infant’s gaze of both eyes collected by the Tobii eye tracker and was used to confirm that the infant was attending the looming stimuli. (MOV 7.29 mb)
VCrL dipole activity. This movie shows VCrL dipole source waveform activity in the O1 region of an 8-month-old infant in response to a medium loom (9.4 m/s2). Activity is shown in slow motion (see running time in milliseconds) for clarity. (MOV 1.13 mb)
VEP surface activity. This movie shows in real-time VEP surface activity in the O1, Oz and O2 region (in blue) in response to a fast loom (21.1 m/s2) for an 8-month-old infant. The same trial is also repeated in slow motion for clarity. (MOV 4.88 mb)
Rights and permissions
About this article
Cite this article
van der Weel, F.R., van der Meer, A.L.H. Seeing it coming: infants’ brain responses to looming danger. Naturwissenschaften 96, 1385–1391 (2009). https://doi.org/10.1007/s00114-009-0585-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-009-0585-y