Abstract
In the process of bioremediation in the soil contaminated by different oil concentrations, the changes in the microbial numbers (bacteria and fungi) and the enzyme (catalase (CAT), polyphenol oxidase (PPO) and lipase) activities were evaluated over a 2-year period. The results showed that the microbial numbers after 2-year bioremediation were one to ten times higher than those in the initial. The changes in the bacterial and the fungal populations were different during the bioremediation, and the highest microbial numbers for bacteria and fungi were 5.51 × 109 CFU g−1 dry soil in treatment 3 (10,000 mg kg−1) in the initial and 5.54 × 105 CFU g−1 dry soil in treatment 5 (50,000 mg kg−1) after the 2-year bioremediation period, respectively. The CAT and PPO activities in the contaminated soil decreased with increasing oil concentration, while the lipase activity increased. The activities of CAT and PPO improved after the bioremediation, but lipase activity was on the contrary. The CAT activity was more sensible to the oil than others, and could be alternative to monitor the bioremediation process.
Similar content being viewed by others
Bioremediation techniques of oil-contaminated soils are based on the metabolism and mineralization of the contaminants. Soils can naturally reduce mobility and bioavailability of oils as they are retained in soil by sorption, precipitation and complexation reactions. This natural attenuation process (natural remediation) can be accelerated by plants and the microorganisms as agents of amendments (Bolan and Duraisamy 2003). Plants could improve activities of microorganisms and provide the nutrients for the degrading microorganisms in soil (Tordoff et al. 2000). Therefore, the application of plants plays an important role in the restoration of oil-contaminated soils.
Oil matters have the toxic effects on soil biota: They can affect key microbial processes (Obbard 2001) and decrease the numbers and activities of soil microorganisms (Maliszweska-Kordybach and Smreczak 2003). Thus, the biological properties of oil-contaminated soils are usually severely affected. However, long-term oil contamination increases the tolerance of bacterial community on oil matters as well as the tolerance of fungi such as arbuscular mycorrhizal (AM) fungi, which can play an important role in the restoration of contaminated ecosystems (Del Val et al. 1999). Meanwhile, it has been reported that microorganisms could respond quickly and adapt to the environmental changes. The detectable changes of soil physical and chemical properties could precede the changes in microbial populations or activity, which could provide an early sign of soil improvement or an early warning of soil degradation (Pankhurst et al. 1995).
The degradation process of hydrocarbons to simple molecules such as water and carbon dioxide involves many chemical reactions including catalytic reaction by special proteins. Because the catalytic reaction plays the central role in hydrocarbon degradation, it is not surprising that focus is now shifting towards using catalytic proteins as potential monitoring tools during bioremediation. Using organic wastes and compost as a source of organic matter and nutrients is common to improve soil physical and chemical properties (Entry et al. 1997), and reduce the need for inorganic fertilizers in the bioremediation (Madejón et al. 2001). Soil biochemical properties may also be improved by organic matter addition (García-Gil et al. 2000; Madejón et al. 2003).
Usually, bioremediation started soon after the different concentration of oil and the amendments were added in the soil. During the bioremediation, the oil was degraded through complex and biochemical process, and the changes of the soil microbial population and enzyme activities could be measured. The aim of this study was to evaluate the changes of soil microbial population and enzyme activities (catalase (CAT), polyphenol oxidase (PPO) and lipase) in oil-contaminated soil during plant and microorganism remediation in field pot experiment.
Materials and Methods
Soils were sampled from a typical sewage-irrigation site in the Shenfu Irrigation Area (southeast of Shenyang), Liaoning Province, China. The sample site had been used exclusively for agricultural activities. The soil samples were taken from the upper layer (0–20 cm). Soil samples were air-dried in a dark room, mixed well, sieved through a 3 mm sieve, and stored at 4°C. The soil characteristics were summarized in Table 1.
The diesel fuel containing alkanes 90.01%, aromatics 0.82%, colloid and asphaltene 0.91% and others 8.26% was purchased from Fushun second oil factory, Fushun, Liaoning Province, China. The plant (Astragalus adsurgens Pall) and microorganisms were selected. The design of this bioremediation experiment was same as the report of Lin et al. (2008).
The dominant degrading microorganisms, including 17 bacterial and fungal strains, used in this study were isolated by Lin’s research group from aged oil-contaminated soil in Shenfu Irrigation Area (Lin et al. 2004). The procedure of microbial culture is as below. 150 mL of liquid culture medium containing 20% (v/v) potato juice was added into a 500 mL conical flask, and autoclaved at 120°C for 20 min. The degrading microorganisms were then inoculated and shaken on a mechanical shaker (120 rpm) at 25°C for 5–7 days. Rice husk and bran (3:1 w/w) were mixed and autoclaved at 120°C for 1 h. The microbial inoculants prepared were then inoculated in a proportion 150 mL culture medium per kg in aseptic conditions. Meanwhile, the water content was adjusted using potato juice to 20–25%. The microbial inoculants were then cultured at 25°C for 5–10 days, in which the main bacteria (9.51 × 1014 CFU g−1) strains were Bacillus, Zoogloea, C2, and C4, and the main fungi (4.39 × 109 CFU g−1) strains included Cunninghamella elegans, Monascus anka, Mucor mandshuricus, B5, B6, B10, Phanerochaete chrysosporium.
A sum of 1.5 kg of unsterilized, air-dried soil was weighed into the ceramic pots. The diesel fuel in acetone solution (50% v/v) was added to the soil bringing the concentrations of 2, 5, 10, 30, 50 g kg−1 of air-dried soil, respectively. After each addition, the soil was carefully mixed to obtain homogeneity (Table 2). The soil–diesel mixture was allowed to stabilize and evaporate for 14 days in the dark room, at a temperature of 18°C in laboratory before planting. Twenty-five A. adsurgens seeds were broadcasted on top of the soil in each pot. Meanwhile, the fertilizer (2% w/w), microbial inoculants (0.5% w/w) and fungi (0.5% w/w) and the water (40% w/w of the soil water holding capacity) were added. When the first leaf developed, the seedlings were thin out, leaving 15 plants in each pot in the first year. Plants were grown for 125 days in a screened growth room (covering with a net in a field) each year, and the plants in the second year were re-growth of the originals. During the growth season, the plants were watered only as needed. Control pots were without diesel fuel application. All pot experiments were performed in triplicates (Lin et al. 2008).
Soil of pot experiments was sampled three times: at initial (before A. adsurgens seeds were sowed), at harvest in the first and second year (after 125 days growth of plant). Some collected soil samples were sieved 2 mm and stored at 4°C keeping its outdoor moisture. The isolation and enumeration of soil microorganisms were performed using the plate-count techniques with nutrient broth agar for bacteria and potato dextrose agar for fungi. Aqueous suspensions of the microbial population of 10 g soil sample were serially diluted. Plates were incubated at 28°C for 2 or 3 days prior to counting colony forming units (CFU).
Other collected soil samples were air-dried, crushed and sieved through a 1 mm sieve and then saved to test. Three kinds of soil enzyme activities were determined as proposed by Guan (1986). CAT activity was determined using potassium permanganate titration method. PPO activity was measured as indicated by colorimetry method. Lipase activity was determined by the method of KOH-ethanol titration. For measuring CAT, 2 g air-dried soil with 40 mL distilled water and 5 mL 0.3% H2O2 was shaken for 20 min (150 n min−1), and then 5 mL 3N H2SO4 was added to stabilize the undecomposed H2O2. The 25 mL filtrate was titrated with 0.1N KMnO4. The results of CAT activity were expressed as mL 0.1N KMnO4 g−1 (20 min)−1. For measuring PPO, 1 g of air-dried soil was incubated for 2 h in water bath at 30°C with 10 mL 1% pyrogallol after shaking, and then 4 mL citric acid (pH 4.5) and phosphoric acid buffer and 35 mL ethyl ether were added successively. The mixture was shaken and extracted for 30 min, and the absorbance of the supernatant was read at 430 nm. The results of PPO activity were from the standard curve which was depended on the content of gallicin and were expressed as mg gallicin g−1 (2 h)−1. For measuring lipase activity, 5 g air-dried soil was firstly treated by 2 mL toluene for 15 min. Secondly, the mixture was incubated for 72 h in the constant-temperature incubator (30°C) after 5 mL distilled water, 5 mL acetate buffer (pH 7.0) and 2.5 mL R-glycicyl butanoate were added. Then, the 10 mL 96% ethanol was added and the mixture was filtered. Finally, 10 mL filtrate was titrated with 0.1N KOH-ethanol. The results of lipase activity were expressed as mL 0.1N KOH g−1 (72 h)−1. During the measurement of these enzymes, the blanks which were composed of distilled water and soil or only substrate solution were measured.
The statistical analyses were carried out between treatments. The difference was calculated using ANOVA and post hoc Tukey test with SPSS Version 13.0 at 95% confidence.
Results and Discussion
Table 3 indicated the changes of microbial numbers in the oil-contaminated soil during the bioremediation. At the beginning of the bioremediation, the microbial numbers increased with the increasing oil concentrations in soils. the highest microbial numbers for bacteria and fungi were 5.51 × 109 CFU g−1 dry soil in treatment 3 (10,000 mg kg−1) in the initial and 5.54 × 105 CFU g−1 dry soil in treatment 5 (50,000 mg kg−1) after the 2-year bioremediation period, respectively. In these five treatments the microbial numbers were higher in more than 2,000 mg kg−1 treatments. The fungal number was the highest in 50,000 mg kg−1 treatment, which was over one order of magnitude than those in other four treatments (Table 3). The increase of microbial numbers might be from the added contaminants which were carbon resources for the microbial growth, and had different effects on bacteria and fungi populations.
The release of exudates and lysates of plant roots could stimulate the microbial growth and influence the diversity of microorganisms especially in rhizosphere (Curl and Truelove 1986). During the two bioremediation periods (i.e. first year and second year), the bacterial population in the control increased consecutively. However, the change in fungal population was not similar to that in bacterial population in the control. The number of fungi decreased in the first bioremediation period, which might be from the nutrient competition with bacteria.
During the first bioremediation period, the bacterial numbers increased one order of magnitude in treatment 1, 300% in treatment 2, and decreased 1 to 2 orders of magnitude in treatment 3, 4 and 5 compared to those in the initial, respectively. During this period, the fungal numbers decreased 1–3 orders of magnitude for all the treatments and the lowest was in treatment 5 (4.90 × 102 CFU g−1 dry soil). The decrease of microbial population might be caused from the toxicity of the medial metabolite, especially for fungi. Thus, in the fist bioremediation period, the degradation of oil matters might be dominant by bacteria.
During the second bioremediation period, the bacterial numbers decreased one order of magnitude in the low concentrations and increased 1 to 2 orders of magnitude in the high concentrations. The fungal numbers were 2 to 3 orders of magnitude higher than those at the end of the first bioremediation period. In the second period, aromatic hydrocarbons as the dominant residues from the first bioremediation period were not degraded by bacteria, but stimulated the growth of fungi. Moreover, due to the oil matters remained greatly after the first bioremediation, the bacterial population was high too.
In field bacteria and fungi exist simultaneously, which could degrade the oil matters and aromatic hydrocarbons in oil-contaminated soil, respectively. The dominant microorganism population might be changed with the biodegradation process. Therefore, selecting the fitful microbial population would be one of the key factors for the successful bioremediation.
During the biochemical process, oxidizing and reducing enzymes in soil have the central role. The enzymes could help to catalyze and transform the organic contaminants, and therefore could be great potential bio-indicators of hydrocarbon removal. When the soil was oil-contaminated, the soil enzymes would be affected and their activities would be changed correspondingly. The application of the joint bioremediation of bacteria and fungi had a positive effect on the soil biological properties. In this study, the joint bioremediation decreased the oil concentrations in the soil during the experimental period. In this study, three kinds of soil enzymes were selected mainly due to their benefit to soil microorganisms and plant roots, and to lead to a better bioremediation in the oil-contaminated soil (Zhou and Song 2004).
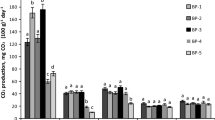
CAT exists in the soil and organisms widely. In general the CAT activity in soil is high. Its role is to catalyze the decomposition of hydrogen peroxide, which is harmful to the organism, into water and oxygen. The CAT activity in oil-contaminated soil was significantly lower than that in control after the first bioremediation period (p < 0.05) shown in Fig. 1. The CAT activities in treatments 3 and 5 were higher than that in treatment 4. It might be related to the effect of the oil on the microorganisms. The oil concentration in treatment 4 (30,000 mg kg−1) might be the threshold concentration, in which more toxicity was caused by the medial metabolites during the biodegradation of the oil matters. After the second bioremediation period, the relationship between the CAT activity and the oil concentrations was just similar to that in the first bioremediation period, but the CAT activity in the second period was higher than that in the first period. The CAT activity in the soil contaminated by the low concentration of oil was similar to that in the control. The enzyme activity in high oil concentration soil increased but only restored to half of the control. The combination of plant and microorganisms could promote CAT activity strongly and restore them.
While the hydrocarbon components of oil were mineralized, the hydroxybenzene would be produced. There was a positive correlation between the PPO activity and the concentration of hydroxybenzene in the soil. Therefore, PPO activities could reflect the bioremediation process in the oil-contaminated soil. The activities of PPO in all treatment soils were significantly lower than that in the control during the first bioremediation period shown in Fig. 2, and the difference among them was not significant (p < 0.05). It suggested that even if the concentration of oil was lower it still could significantly weaken the PPO activity in soil. However, the PPO activities in all treatments after the second bioremediation period were obviously higher than those after the first period bioremediation, except that in the treatment 3. The highest PPO activity appeared in the highest oil-concentration soil after two-period bioremediation. The hydroxybenzene produced during oil biodegradation might stimulate plant and microbe to secrete PPO, which enhanced the oxidization of the hydroxybenzene. This was consistent with the results of this study. The PPO activity decreased for medial concentrations (5,000–10,000 mg kg−1 treatments).
The lipase produced by a large variety of microorganisms, animals and plants, could degrade lipids in glycerine and fatty acids. The measurement of the lipase activity might represent a valid tool in monitoring the biodegradation of organic pollutants. Figure 3 showed the change of lipase activity in the soil. With the increased oil concentration, the lipase activity was enhanced except that in treatment 2, which was similar to some studies (Margesin et al. 2000). The lipase activity arrived the peak value in treatment 3 (19% higher than that in control) in the first bioremediation period. The difference of the lipase activity among these treatments (except the treatment 5, in which the lipase activity was 24% higher than that in the control) was not significant (p < 0.05) in the second bioremediation period. However, the lipase activities in the second bioremediation period was significant (p < 0.05) lower than those in the first bioremediation period. The changes of lipase activity indicated that the grease in soil enhanced the growth of microbe and improved the metabolism of total petroleum hydrocarbons.
Table 4 showed the CAT, PPO, lipase activities and their statistic characteristics during the bioremediation. In the oil-contaminated soil the average lipase activity was 15.41 and 9.44 in the first and second bioremediation period respectively, and was the highest followed by PPO and CAT activities in each bioremediation period. In treatment 3, the lipase activity was up to the maximum value of 17.2, while the CAT activity was the lowest only 0.05 in the first bioremediation period. There were different enzyme activities which appeared in different bioremediation period. CAT and PPO activities in the first bioremediation period were lower than those in the second bioremediation period, the change of which was opposite to that of the lipase activity. The coefficients of variation of the CAT and PPO activities had the similar trend, and the difference of those of the lipase activity was not significant between these two bioremediation periods (p < 0.05).
The changes of these enzymes among these treatments could be expressed by their coefficients of variation. The highest coefficient of variation showed that oil concentrations affected the change of the enzyme activity significantly. Thus, it can be seen that the effect of oil concentration on the lipase activity was not significant; in contrast, the effect of oil concentration on the CAT activity was significant in the first period bioremediation. The coefficient of variation of the CAT activity was up to 1.01. Hence, the CAT activity might be sensitive to the oil concentration in the soil and could be used to monitor soil contamination.
Joint remediation, especially the synergy of plant and microbe, would be very effective to improve the quality of the oil-contaminated soil. The microbial populations after 2-year bioremediation were 1–10 times higher than those in the initial in the oil-contaminated soil. The CAT and PPO activity in the contaminated soil decreased with the oil concentration increasing. However, the lipase activity increased with oil concentration increasing. The activity of CAT and PPO improved during the bioremediation, but lipase activity was in opposition. Generally the effect of oil concentration on the enzyme activity was not significant during the bioremediation. The CAT activity to the oil was more sensible than other enzymes, and could be alternative to monitor the contamination or bioremediation process.
References
Bolan NS, Duraisamy VP (2003) Role of inorganic and organic soil amendments on immobilization and phytoavailability of heavy metals: a review involving specific case studies. Aust J Soil Res 41:533–555
Curl EA, Truelove B (1986) The rhizophere. Springer, New York, pp 9–54
Del Val C, Barea JM, Azcón-Aguilar C (1999) Assessing the tolerance to heavy metals of arbuscular mycorrhizal fungi isolated from sewage sludge-contaminated soils. Appl Soil Ecol 11:261–269
Entry JA, Wood BH, Edwards JH, Wood CW (1997) Influence of organic by products and nitrogen source on chemical and microbiological status of an agricultural soil. Biol Fertil Soils 24:196–204
García-Gil JC, Plaza C, Soler-Rovira P, Polo A (2000) Longterm effects of municipal solid waste compost application on soil enzyme activities and microbial biomass. Soil Biol Biochem 32:1907–1913
Guan SM (1986) Soil enzyme and research method[M]. Agriculture Press, Beijing, pp 274–339
Lin X, Li P, Zhou QX, Xu HX, Zhang HR (2004) Microbial changes in rhizospheric soils contaminated with petroleum hydrocarbons after bioremediation. J Environ Sci 16:987–990
Lin X, Li XJ, Li PJ, Li F, Zhang L, Zhou QX (2008) Evaluation of plant–microorganism synergy for the remediation of diesel fuel contaminated Soil. Bull Environ Contam Toxicol 81:19–24
Madejón P, Murillo JM, Marañón T, Cabrera F, López R (2001) Elementos traza en gramíneas afectadas por el vertido tóxico de las minas de Aznalcóllar. Invest Agr Prod Prot Veg 16:429–446
Madejón E, Burgos P, López R, Cabrera F (2003) Agricultural use of three organic residues: effect on orange production and on properties of a soil of the “Comarca Costa de Huelva” (SW Spain). Nutr Cycl Agroecosys 65:281–288
Maliszweska-Kordybach B, Smreczak B (2003) Habitat function if agricultural soils as affected by heavy metals and polycyclic aromatic hydrocarbons contamination. Environ Int 28:719–728
Margesin R, Zimmerbauer A, Schinner F (2000) Monitoring of bioremediation by soil biological activities. Chemosphere 40:339–346
Obbard P (2001) Ecotoxicological assessment of heavy metals in sewage sludge amended soils. Appl Geochem 16:1405–1411
Pankhurst CE, Hawke BG, McDonald HJ, Kirkby CA, Buckerfield JC, Michelsen P, O’Brien KA, Gupta VVSR, Doube BM (1995) Evaluation of soil biological properties as potential bioindicators of soil health. Aust J Exp Agr 35:1015–1028
Tordoff GM, Baker AJM, Willis AJ (2000) Current approaches to the revegetation and reclamation of metalliferous wastes. Chemosphere 41:219–228
Zhou QX, Song YF (2004) Principles and methods of contaminated soil remediation. Science Press, Beijing, pp 556–560
Acknowledgments
This research was supported by funds provided by Innovative Program of The Chinese Academy of Sciences (KZCX2-YW-446), the National High Technology Research and Development Program of China (2007AA06A405), National Natural Science Foundation of China (20807029), and National Basic Research Program of China (2004CB418506).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, X., Li, X., Sun, T. et al. Changes in Microbial Populations and Enzyme Activities During the Bioremediation of Oil-Contaminated Soil. Bull Environ Contam Toxicol 83, 542–547 (2009). https://doi.org/10.1007/s00128-009-9838-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-009-9838-x