Abstract
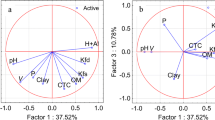
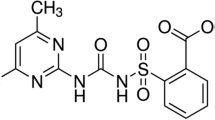
Soil sorption, described as logKOC (the logarithm of the soil/water partition coefficient normalized to organic carbon), was modeled using the augmented multivariate image analysis applied to quantitative structure–property relationship method for a series of 11 carboxylic acid herbicides. The statistical model was found to be highly predictive and reliable to estimate logKOC of other persistent organic pollutants in the soil, which are analogues of the carboxylic acids used in the QSPR model. The QSPR model derived from images corresponding to the chemical structures of the 11 herbicides is superior to the uniparameter model based on the octanol/water partition coefficient (logP) and, in addition, a pattern recognition model was built using principal component analysis. This model allowed clustering and separating compounds with low/moderate soil sorption from those with moderate/high soil sorption (compounds with the aryloxy function) using the second principal component.



Similar content being viewed by others
References
Brown RD, Martin YC (1997) The information content of 2D and 3D structural descriptors relevant to ligand-receptor binding. J Chem Inf Comput Sci 37:1–9
Corsonlini S, Ademollo N, Romeo T, Greco S, Focardi S (2005) Persistent organic pollutants in edible fish: a human and environmental health problem. Microchem J 79:115–123
Domingo JL (2004) Human exposure to polybrominated diphenyl ethers through the diet. J Chromatogr A 1054:321–326
Estrada E, Molina E, Perdomo-López I (2001) Can 3D structural parameters be predicted from 2D (topological) molecular descriptors? J Chem Inf Comput Sci 41:1015–1021
Freitas MP, Ramalho TC (2013) Employing conformational analysis in the molecular modeling of agrochemicals: insights on QSAR parameters of 2,4-D. Ciênc Agrotechnol 37:485–494
Freitas MR, Matias SVBG, Macedo RLG, Freitas MP, Venturin N (2013) Augmented multivariate image analysis applied to quantitative structure–activity relationship modeling of the phytotoxicities of benzoxazinone herbicides and related compounds on problematic weeds. J Agric Food Chem 61:8499–8503
Freitas MR, Matias SVBG, Macedo RLG, Freitas MP, Venturin N (2014) Three-parameter modeling of the soil sorption of acetanilide and triazine herbicide derivatives. Bull Environ Contam Toxicol 92:143–147
Giesy JP, Ludwig JP, Tillitt DE (1994) Deformities in birds of the Great-Lakes region assigning causality. Environ Sci Technol 28:A128–A135
Kavlock RJ, Daston GP, Derosa C, Fenner-Crisp P, Gray LE, Kaattari S, Lucier G, Luster M, Mac MJ, Maczka C, Miller R, Moore J, Rolland R, Scott G, Sheehan DM, Sinks T, Tilson HA (1996) Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the US EPA sponsored workshop. Environ Health Perspect 104:715–740
Kelce WR, Stone CR, Laws SC, Gray LE, Kemppainen JA, Wilson EM (1995) Persistent DDT metabolite P, P’-DDE is a potent androgen receptor antagonist. Nature 375:581–585
Mackay D, Shiu W-Y, Ma K-C (1997) Illustrated handbook of physical-chemical properties and environmental fate for organic chemicals. Lewis Publishers, New York
Mitra I, Saha A, Roy K (2010) Exploring quantitative structure–activity relationship studies of antioxidant phenolic compounds obtained from traditional Chinese medicinal plants. Mol Simul 36:1067–1079
Nunes CA, Freitas MP (2013) Introducing new dimensions in MIA-QSAR: a case for chemokine receptor inhibitors. Eur J Med Chem 62:297–300
Ratcliffe DA (1967) Decrease in eggshell weight in certain birds of prey. Nature 215:208–210
Ratcliffe DA (1970) Changes attributable to pesticides in egg breakage frequency and eggshell thickness in some British birds. J Appl Ecol 7:67–115
Tan X, Calderon-Villalobos LIA, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N (2007) Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446:640–645
Acknowledgments
Authors are grateful to FAPEMIG, CNPq and CAPES for the financial support of this research, as well as for the fellowships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Freitas, M.R., Freitas, M.P. & Macedo, R.L.G. Aug-MIA-QSPR Modeling of the Soil Sorption of Carboxylic Acid Herbicides. Bull Environ Contam Toxicol 93, 489–492 (2014). https://doi.org/10.1007/s00128-014-1356-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-014-1356-9