Abstract
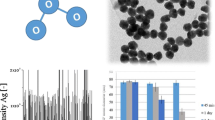
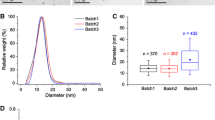
Methods are needed to prepare stable suspensions of engineered nanoparticles in aqueous matrixes for ecotoxicity testing and ecological risk assessments. We developed a novel method of preparing large volumes of silver nanoparticles (AgNP) in suspension using a commercially available rotor–stator dispersion mill. AgNP in powder form (PVP capped, 30–50 nm) was suspended in deionized water and natural lake water at 1 g/L and the addition of 0.025% (w/v) gum arabic (GA) increased stability over 2 weeks after preparation. The concentrations of total and dissolved Ag in the suspensions did not change significantly over this period. Analysis of hydrodynamic diameters of the major peaks in suspension using dynamic light scattering showed that suspensions prepared with GA were stable, and this was confirmed by single-particle ICP-MS analysis. This method for dispersing AgNPs provides an inexpensive, yet reliable method for preparing suspensions for toxicity testing and ecosystem level studies of the fate and biological effects of AgNPs in aquatic ecosystems.




Similar content being viewed by others
References
Batley GE, Kirby JK, McLaughlin MJ (2013) Fate and risks of nanomaterials in aquatic and terrestrial environments. Acc Chem Res 46:854–862
Bilberg K, Malte H, Wang T, Baatrap E (2010) Silver nanoparticles and silver nitrate cause respiratory stress in Eurasian perch (Perca fluviatilis). Aquat Toxicol 96:159–165.
Cheng Y, Yin L, Lin S, Wiesner M, Bernhardt E, Liu J (2011) Toxicity reduction of polymer-stabilized silver nanoparticles by sunlight. J Phys Chem C 115:4425–4432
Cleveland D, Long SE, Pennington PL, Cooper E, Fulton MH, Scott GI, Brewer T, Davis J, Petersen EJ, Wood L (2012) Pilot estuarine mesocosm study on the environmental fate of silver nanomaterials leached from consumer goods. Sci Total Environ 421–422:267–273
Furtado LM, Hoque ME, Mitrano DF, Ranville JF, Cheever B, Frost PC, Xenopoulos MA, Hintelmann H, Metcalfe CD (2014) The persistence and transformation of silver nanoparticles in littoral lake mesocosms monitored using various analytical techniques. Environ Chem 11:419–430
Furtado LM, Cheever B, Xenopoulus MA, Frost PC, Metcalfe CD, Hintelmann H (2015) Fate of silver nanoparticles in freshwater lake mesocosms. Environ Sci Technol 49:8441–8450
Furtado LM, Bundschuh M, Metcalfe CD (2016) Monitoring the fate and transformation of silver nanoparticles in natural waters. Bull Environ Contam Toxicol 97:449–455
Gao J, Youn S, Hovsepyan A, Llaneza VI, Wang Y, Bitton G, Bonzongo J-CJ (2009) Dispersion and toxicity of selected manufactured nanomaterials in natural river water samples: Effects of water chemical composition. Environ Sci Technol 43:3322–3328
Garner KL, Suh S, Lenihan HS, Keller AA (2015) Species sensitivity distributions for engineered nanoparticles. Environ Sci Technol 49:5753–5759
Hadioui M, Leclerc S, Wilkinson K (2013) Multimethod quantification of Ag+ release from nanosilver. Talanta 2013:15–19
Handy RD, Geert C, Ferrnandes TF et al (2012) Ecotoxicity test methods for engineered nanomaterials: practical experiences and recommendations from the bench. Environ Toxicol Chem 31:15–31
Juby KA, Dwivedi C, Kumar M, Kota S, Misra HS, Bajaj PN (2012) AgNP-loaded PVA/gum acacia hydrogel: Synthesis, characterization and antibacterial study. Carbohydr Polym 89:906–913
Kittler S, Greulich C, Diendorf J, Koller M, Epple M (2010) Toxicity of AgNP increases during storage because of slow dissolution under release of silver ions. Chem Mater 22:4548–4554
López-Miranda A, López-Valdivieso A, Viramontes-Gamboa G (2012) AgNP synthesis in aqueous solutions using sulfite as reducing agent and SDS as stabilizer. J Nanopart Res 14:1–11
Lowry GV, Espinasse BP, Badireddy AR et al (2012) Long-term transformation and fate of manufactured Ag nanoparticles in a simulated large scale freshwater emergent wetland. Environ Sci Technol 46:7027–7036
Mallevre F, Alba C, Milne C, Gillespie S, Fernandes TF, Aspray TJ (2016) Toxicity testing of pristine and aged silver nanoparticles in real wastewaters using bioluminescent Pseudomonas putida. Nanomaterials 6:49–62.
Martin JD, Colson T-L, Langlois VS, Metcalfe CD (2017) Biomarkers of exposure to nanosilver and silver accumulation in yellow perch (Perca flavescens). Environ Toxicol Chem. doi:10.1002/etc.3644
Murray L, Rennie M, Enders E, Pleskach K, Martin J (2017) Effect of nanosilver on cortisol release and morphometrics in rainbow trout (Oncorhynchus mykiss). Environ Toxicol Chem. doi:10.1002/etc.3691
Nowack B, Ranville J, Diamond S, Gallego-Urrea J, Metcalfe CD, Rose J, Horne N, Koelmans AA, Klaine SJ (2012) Potential scenarios for nanomaterial release and subsequent alteration in the environment. Environ Toxicol Chem 31:50–59
OECD (2012) Guidance on sample preparation and dosimetry for the safety testing of manufactured nanomaterials, Series on the Safety of Manufactured Nanomaterials No. 36, Organization of Economic Cooperation and Development, Environment Directorate, Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology in Paris, ENV/JM/MONO(2012) 40, 93 p
Olenin AY, Krutyakov YA, Kudrinskii AA, Lisichkin GV (2008) Formation of surface layers on silver nanoparticles in aqueous and water-organic media. Colloid J 70:71–76
Peijnenburg WJGM, Baalousha M, Chen J et al (2015) A review of the properties and processes determining the fate of engineered nanomaterials in the aquatic environment. Crit Rev Environ Sci Technol 45:2084–2134
Pham CH, Yi J, Gu MB (2012) Biomarker gene response in male medaka (Oryzias latipes) chronically exposed to silver nanoparticles. Ecotoxicol Environ Safety 78:239–245.
Sabri N, Hanna K, Yargeau V (2012) Chemical oxidation of ibuprofen in the presence of iron species at near neutral pH. Sci Total Environ 427–428:382–389
Schaumann GE, Philippe A, Bundschuh M et al (2015) Understanding the fate and biological effects of Ag- and TiO2-nanoparticles in the environment: the quest for advanced analytics and interdisciplinary concepts. Sci Total Environ 535:3–19
Schultz AG, Boyle D, Chamot D, Ong K, Wilkinson KJ, McGeer JC, Sunahara G, Goss GG (2014) Aquatic toxicity of manufactured nanomaterials: challenges and recommendations for future toxicity testing. Environ Chem 11:207–226
Telgmann L, Metcalfe CD, Hintelmann H (2014) Rapid size characterization of silver nanoparticles by single particle ICP-MS and isotope dilution. J Anal Atom Spectrom 29:1265–1272
The Project on Emerging Nanotechnologies. Consumer products inventory. http://www.nanotechproject.org/cpi. Accessed July 5 2016)
Vance ME, Kuiken T, Vejerano EP, McGinnis SP, Hochella MF Jr, Rejeski D, Hull MS (2015) Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J Nanotechnol 6:1769–1780
Wang D, Xie T, Peng Q, Li Y (2008) Ag, Ag2S, and Ag2Se nanocrystals: synthesis, assembly, and construction of mesoporous structures. J Am Chem Soc 130:4016–4022
Acknowledgements
The authors thank M. E. Hoque and B. Seaborn at Trent University for their technical assistance, L. Shen and V. Yargeau at McGill University for the TEM photomicrographs, and T. Kritzer at Kady® International for providing advice on how to optimize mill parameters for preparing AgNP suspensions
Funding
Funding was provided by the Natural Sciences and Engineering Research Council (NSERC) of Canada through the Strategic Grants Program (#413230-2011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martin, J.D., Telgmann, L. & Metcalfe, C.D. A Method for Preparing Silver Nanoparticle Suspensions in Bulk for Ecotoxicity Testing and Ecological Risk Assessment. Bull Environ Contam Toxicol 98, 589–594 (2017). https://doi.org/10.1007/s00128-017-2067-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-017-2067-9