Abstract
Objective
To study the effect of non-exertional heatstroke on serum procalcitonin (PCT) levels.
Design
Cohort study.
Setting
The emergency and intensive care departments of two academic tertiary-care hospitals, Paris, France
Patients
A total of 53 patients with defined heatstroke attending the emergency department and/or the intensive care unit during the August 2003 heat wave in France.
Interventions
None.
Measurements
Serum PCT measurement using a sensitive assay and vital and routine biological variables on arrival of patients presenting with classic heatstroke. Thirty-day mortality was recorded.
Results
Among the 53 patients included, 14 (26%) were admitted to an intensive care unit (ICU). At 30 days, 24 patients (45%) had died. Median PCT value was 0.58 μg/l (95% confidence interval 0.16–1.61) and 31 (58%) patients had PCT above 0.2 μg/l (PCT+). Temperature above or equal to 40 °C was the only variable significantly associated with fatal outcome. Median PCT values were 1.4 μg/l (0.16–4.71) and 0.18 μg/l (0.12–1.61) in the group of deceased and surviving patients respectively (p = 0.22). All patients admitted in ICU had elevated PCT values. Patients PCT+ initially presented with a more pronounced systemic inflammatory response. Microbiologically or clinically documented infection was not more frequent in PCT+ group.
Conclusion
High serum PCT levels can be observed in heatstroke without any concomitant documented bacterial infection. The PCT is not a valid mortality predictor in heatstroke but could be an indicator of the severity of illness. Heatstroke could represent a model of a “non-septic” pathway of PCT synthesis.
Similar content being viewed by others
Introduction
Heatstroke is a life-threatening disease characterized by a body temperature above 40 °C associated with neurologic symptoms [1]. Classic (also called environmental or non-exertional) heatstroke is a climatic-induced illness that can develop during heat waves among heat-exposed populations. Classic heatstroke exhibits an epidemic pattern and may lead hundreds of hyperthermic patients to emergency departments [2]. Conversely, exertional heatstroke may complicate an unusual strenuous exercise conducted in heat atmosphere.
Management of heatstroke epidemic by emergency teams has two major diagnostic objectives: the identification of the more critically ill patients and the detection of those who may benefit from antibiotics, as up to 50% of heatstroke patients have concomitant bacterial infections [3]. The predisposing factors usually accepted to explain concurrent infection during heatstroke include altered immune status, endotoxinemia, and/or bacterial translocation due to gut ischemia and coma contributing to inhaled pneumonia. Moreover, infection may alter heatstroke outcome by contributing to dehydratation, thermal load, and multiorgan failure. Procalcitonin (PCT) is a biological marker that has been reported to be useful for the diagnosis and prognosis of bacterial infection in febrile patients [4–7]. In the only study published to date on PCT and heatstroke, the authors reported that serum PCT concentrations were systematically increased in classic heatstroke particularly among survivors [8].
During the French heat wave in 2003, we received a large number of patients with classic heatstroke and thus were able to determine PCT levels in several of these patients. The aim of our study was to study the effect of classic (non-exertional) heatstroke on serum PCT, and to test if PCT was either a valid prognostic biomarker in this context, and/or a diagnostic marker of concomitant infection.
Patients and methods
From the 1 to the 14 August 2003, France, and especially the Paris area, experienced an unusual heat wave with thousands of patients suffering from heat-related illness presenting to the emergency departments of Assistance Publique-Hôpitaux de Paris (AP-HP) [2]. In the present study, patients were included if: (a) they presented with classic heatstroke, from 1 to 14 August 2003, to one of the 13 emergency departments of AP-HP; and (b) a frozen serum aliquot drawn in emergency room or a PCT measurement at entry in the emergency room was available. Classic heatstroke was defined as a patient with body temperature > 40 °C (tympanic measurement using an infrared tympanic thermometer) or > 39 °C in cases where pre-hospital cooling had been initiated, associated with neurologic symptoms during the duration of the heatwave, and out of the context of an unusual strenuous exercise. Infection was defined by growth of bacteria in blood culture and/or in a sterile fluid (e.g., urine sample), or by a clinically documented source of infection (e.g., pneumonia was defined as the presence of new pulmonary infiltrate on chest radiograph associated with acute respiratory symptoms). The study was approved by the Ethics Committee of our hospital (Comité de Protection des Personnes Pitié-Salpêtrière, Paris). Waived informed consent was authorized because routine care of the patient was not modified.
PCT assay
All samples were drawn at arrival in the emergency room. For serum PCT measurement, we used a time-resolved amplified cryptate emission (TRACE) technology assay (Kryptor PCT, Brahms, Hennigsdorf, Germany). This assay is based on a polyclonal antibody against calcitonin and a monoclonal antibody against katacalcin, which bind to the calcitonin and katacalcin sequence of precursor molecules. This assay has an optimized functional sensitivity of 0.06 μg/l. In healthy volunteers, normal PCT levels are under 0.1 μg/l. We chose a 0.2-μg/l cutoff value as it was reported to be the most effective threshold to diagnose bacterial infections in emergency-department setting [6, 7].
Statistical analysis
Data are expressed as mean ± standard deviation (SD) or median and its 95% confidence interval (CI) for non-Gaussian distribution. Comparison of two means was performed using the Student's t-test, comparison of two medians using the Mann–Whitney test, and comparison of two proportions using the Fisher exact method.
All statistical comparisons were two-tailed and a p-value of less than 0.05 was required to reject the null hypothesis. Statistical analysis was performed using a computer and NCSS 2001 software (Statistical Solutions Ltd, Corke, Ireland).
Results
Among the 13 emergency departments screened during the study period, only two centers had the biological material requested available. One was conducting a prospective observational study on PCT in febrile adult patients [7] and the other had frozen sera samples available, taken in emergency room, from heatstroke patients. In the study design of the first emergency setting, the physicians were blinded to PCT values [7].
A total of 53 patients were finally included, all of whom met classic heatstroke criteria. There were 22 males and 31 females, mean age 74 ± 18 years (extremes: 26–97 years). The mean temperature was 40.0 ± 1.1 °C (extremes: 39.0–42.2 °C). Thirty-nine patients (74%) were admitted to a medical-bed and 14 patients (26%) to the intensive care unit (ICU). The ICU admissions may have been restricted due to the limited ICU beds available because of the summer period of study and overcrowded ICU due to heatstroke epidemic. The main indication for ICU admission was neurological (coma), although 9 of 14 ICU-admitted patients also presented with concomitant respiratory, cardiovascular and renal failures at entry. At 30-day follow-up, 24 patients (45%) had died (including 8 initially admitted to ICU), 12 of them before day 8. Causes of death comprised coma (13 patients), multiorgan failure (10 patients) and ventilator-associated pneumonia (1 patient). All patients underwent cooling techniques comprising fan and the application to the skin of ice and/or sheets wet in cold water. Forty-seven patients (88%) presented with concurrent medical conditions. Twenty-four patients (45%) had a clinically documented source of infection. All patients had blood cultures performed: 10 patients (19%) showed growth of microorganisms in blood cultures (Staphylococcus aureus: 2; coagulase-negative Staphylococcus sp: 4; Enterococcus sp: 1; Streptococcus sanguis: 1; Candida albicans and Pseudomonas aeruginosa: 1; Klebsiella pneumoniae: 1). Median PCT value in the cohort was 0.58 μg/l (0.16–1.61; extremes: 0.1–49 μg/l). Twenty-two patients (41%) had PCT levels below the 0.2-μg/l threshold. Median PCT levels were 0.65 μg/l (0.12–2.54) and 0.48 μg/l (0.15–6.80), respectively, in patients with or without microbiologically documented infection (p = 0.73, NS). Median C-reactive protein value was 8 mg/l (4–14; extremes: 1–191 mg/l) and did not differ significantly between deceased and alive groups (8 (3–14) vs. 8 (2–27), respectively, p = 0.78; Table 1).
Variables associated with outcome
The group of patients alive at 30-day follow-up (n = 29) was compared for qualitative and quantitative variables with the group of deceased patients (n = 24). The results are summarized in Fig. 1 and Table 1. Any quantitative variable was significantly associated with fatal outcome. The only qualitative variable significantly associated with fatal outcome was a temperature above or equal to 40 °C. The PCT was not significantly different between groups, although there was a trend toward higher PCT values in deceased patients (1.4 μg/l (0.16–4.71) vs. 0.18 μg/l (0.12–1.61) in alive group, p = 0.22; Fig. 1; Table 1).
Variables associated with a raised serum PCT level
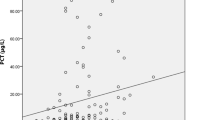
The group of patients with elevated serum PCT values (PCT+, ≥ 0.2 μg/l, n = 31) was compared for qualitative and quantitative variables with the group of patients with negative PCT values (PCT−, < 0.2 μg/l, n = 22). The results are summarized in Table 2. PCT+ patients presented with significantly higher temperature, heart rate, total white blood cell, neutrophil count and creatinine and with a lower diastolic arterial blood pressure (Table 2). Patients PCT+ were more frequently women. They were significantly more likely to be admitted to ICU and to receive antibiotics, although PCT results were not available at the time of medical care (Table 2). Conversely, the presence of microbiologically or clinically documented infection was not significantly different between groups (Fig. 2; Table 2).
Individual values of procalcitonin (PCT) in the groups of heatstroke patients with or without microbiologically documented infection. Data are represented in semi-logarithmic scale. Each dark circle indicates a patient. Dark squares represent medians (CI 95%) and dashed line the 0.2 μg/l threshold for PCT
Discussion
Heatstroke is a life-threatening illness with a complex pathophysiology sharing some similarities with sepsis [1]. Patients with heatstroke usually present clinically with systemic inflammatory response syndrome (SIRS) criteria. The physicians who take care of these hyperthermic patients have two major problems to resolve: the first is to rule out the hypothesis of an infectious disease before diagnosing heatstroke. Moreover, as up to 50% of patients suffering from heatstroke have concomitant bacterial infections [3], the identification of those who actually justify treatment with antibiotics is particularly difficult. The second problem is to identify the more critically ill patients who may benefit from ICU admission. As PCT was reported to be a diagnostic and prognostic marker of bacterial infection among febrile patients [5–7], we studied its usefulness in heatstroke and observed that PCT was neither a good indicator of associated infection in heatstroke nor a valid prognostic marker, although an elevated PCT value seemed to be correlated with a more critical illness.
In this study, we report a 45% mortality rate. This apparently high mortality rate is in accordance with previous published studies conducted in either Western or Middle-Eastern countries and ranging from 40 to 60% [3, 9–11]. The epidemic mode of presentation of heat-related diseases with a particularly high attack rate, together with the reduced health care available during the summer period and the absence of standardized cooling protocol, may have contributed to this high mortality rate [2].
We report that 58% of heatstroke patients had elevated PCT values and that PCT rise was not specific for infection in this context (Fig. 2; Table 2). Interestingly, PCT+ patients were more prone to receive antibiotics, although the result of PCT measurement was not available at this time. One explanation could be that PCT+ patients presented more frequently with a clinical and biological sepsis-like pattern than the PCT- group. Indeed, PCT+ patients had significantly higher temperature, heart rate, total white blood cell, neutrophil count, and creatinine, all variables that may indicate a more pronounced systemic inflammatory response than PCT- patients (Table 2). Our data are in accordance with the only study on PCT and heatstroke published to date that reported serum PCT rise in classic heatstroke [8]. Surprisingly, in the present study median CRP levels were low (8 mg/l), although all patients presented with SIRS. Indeed, CRP levels are known to raise non-specifically in most inflammatory syndromes, contrary to PCT which was reported to be more sensitive and specific with regard to the bacterial origin of inflammation [4–7]. In the particular context of heatstroke, the stimulation of CRP synthesis seems to be lesser than in other inflammatory situations [11].
Heatstroke could represent a non-septic pathway for PCT synthesis. On one hand, some studies reported an elevated plasma level of inflammatory cytokines (tumor necrosis factor α (TNF-α), interleukin-1β, interferon-γ) in classic heatstroke [12–14]. The TNF-α precedes PCT detection in serum after endotoxin injection in normal subjects and injection of TNF-α can stimulate PCT synthesis in animal models [15, 16]. It is a matter of speculation whether sepsis and heatstroke share TNF-α as a common inducer of PCT. On the other hand, a more recent study reported that TNF-α and interleukin-1β were below the detection limit in an experimental baboon model of heatstroke [17]. More complex inflammatory and perhaps coagulation responses of the host may contribute to the “non-septic” PCT elevation in heatstroke, as has been observed in Kawasaki disease, hemophagocytic syndrome, or in the early post-traumatic period, all situations characterized by a major and uncontrolled inflammatory response [7, 18–20].
Conversely, we cannot confirm that heatstroke patients with elevated PCT values had a better outcome, as Nylen et al. reported [8]. In our study, all 14 patients admitted to intensive care unit had elevated PCT values (Table 2). None of the patients with low PCT levels (< 0.2 μg/l) was admitted to ICU. Although not reaching statistical significance, there was a trend toward higher median PCT values in deceased patients and more deaths in PCT+ group (Fig. 1; Tables 1, 2). Overall, PCT in heatstroke might be an indicator of the severity of the illness, as in sepsis. This could be of major importance, since the selection of the more critically ill patients is particularly difficult in the context of heatstroke epidemic because of associated overcrowded emergency departments; however, the usefulness of PCT as a screening biological tool in heatstroke has yet to be confirmed in larger studies.
This study has some limitations. Firstly, although larger than the first study by Nylen et al. [8], the size of the cohort was small. Heatstroke is a rare disease in Europe, and because of the excess emergency visits related to an unpredictable heatwave, emergency departments were overcrowded and suffered from disorganization. This did not allow us to conduct a systematic prospective study. Secondly, we did not study the kinetics of PCT levels in these patients. Finally, we did not study circulating levels of inflammatory and anti-inflammatory cytokines, which could have been useful in determining the host inflammatory pattern response to heatstroke.
Conclusion
Heatstroke can be associated with elevated serum PCT values irrespective of the presence of concomitant bacterial infection. Heatstroke patients with high PCT levels may have a more critical disease, although PCT did not correlate with mortality. Larger studies are required to determine if PCT is able to identify the more critical heatstroke patients who may benefit from ICU admission and the optimal threshold in this condition. Heatstroke may represent a model of a “non-septic” pathway of PCT synthesis, and further studies, including circulating cytokines measurements, would be able to determine the mechanisms involved in this inflammatory disease.
References
Bouchama A, Knochel JP (2002) Heat stroke. N Engl J Med 346:1978–1988
Dhainaut JF, Claessens YE, Ginsburg C, Riou B (2004) Unprecedented heat-related deaths during the 2003 heat wave in Paris: consequences on emergency departments. Crit Care 8:1–2
Dematte JE, O'Mara K, Buescher J, Whitney CG, Forsythe S, McNamee T, Adiga RB, Ndukwu IM (1998) Near-fatal heat stroke during the 1995 heat wave in Chicago. Ann Intern Med 129:173–181
Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J (2004) Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis 39:206–217
Delevaux I, André M, Colombier M, Albuisson E, Meylheuc F, Bègue R-J, Piette JC, Aumaître O (2003) Can procalcitonin measurement help in differentiating between bacterial infection and other kinds of inflammatory processes? Ann Rheum Dis 62:337–340
Hausfater P, Garric S, Ben Ayed S, Rosenheim M, Bernard M, Riou B (2002) Usefulness of procalcitonin as a marker of systemic infection in emergency department patients: a prospective study. Clin Infect Dis 34:895–901
Hausfater P, Juillien G, Madonna-Py B, Haroche J, Bernard M, Riou B (2007) Serum Procalcitonin measurement as diagnostic and prognostic marker in febrile adult patients presenting to the emergency department. Crit Care 11:R60
Nylen ES, Al Arifi A, Becker KL, Snider RH Jr, Alzeer A (1997) Effect of classic heatstroke on serum procalcitonin. Crit Care Med 25:1362–1365
Misset B, De Jonghe B, Bastuji-Garin S, Gattolliat O, Boughrara E, Annane D, Hausfater P, Garrouste-Orgeas M, Carlet J (2006) Mortality of patients with heatstroke admitted to intensive care units during the 2003 heat wave in France: a national multiple-center risk-factor study. Crit Care Med 34:1087–1092
Bouchama A, Dehbi M, Mohamed G, Matthies F, Shoukri M, Menne B (2007) Prognostic factors in heat wave-related deaths. Arch Intern Med 167:2170–2176
Argaud L, Ferry T, Le Q-H, Marfisi A, Ciorba D, Achache P, Ducluzeau R, Robert D (2007) Short- and long-term outcomes of heastroke following the 2003 heat wave in Lyon, France. Arch Intern Med 167:2177–2183
Robins HI, Kutz M, Wiedemann GJ, Katschinski DM, Paul D, Grosen E, Tiggelaar CL, Spriggs D, Gillis W, d'Oleire F (1995) Cytokine induction in human by 41.8 degrees C whole body hyperthermia. Cancer Lett 97:195–201
Chang DM (1993) The role of cytokines in heatstroke. Immunol Invest 22:553–561
Bouchama A, al-Sedairy S, Siddiqui S, Shail E, Rezeig M (1993) Elevated pyrogenic cytokines in heatstroke. Chest 104:1498–1502
Whang KT, Vath SD, Becker KL, Snider RH, Nylen ES, Muller B (2000) Procalcitonin and proinflammatory cytokine interactions in sepsis. Shock 14:73–78
Dandona P, Nix D, Wilson MF, Aljada A, Love J, Assicot M, Bohuon C (1994) Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab 79:1605–1608
Bouchama A, Ollivier V, Roberts G, Al Mohanna F, de Prost D, Eldali A, Saussereau E, El-Sayed R, Chollet-Martin S (2005) Experimental heatstroke in baboon: analysis of the systemic inflammatory response. Shock 24:332–335
Gendrel D, Raymond J, Coste J, Moulin F, Lorrot M, Guérin S, Ravilly S, Lefèvre H, Royer C, Lacombe C, Palmer P, Bohuon C (1999) Comparison of procalcitonin with C-reactive protein, interleukin 6 and interferon-alpha for differentiation of bacterial vs. viral infections. Pediatr Infect Dis J 18:875–881
Mimoz O, Benoist JF, Edouard AR, Assicot M, Bohuon C, Samii K (1998) Procalcitonin and C-reactive protein during the early posttraumatic systemic inflammatory response syndrome. Intensive Care Med 24:185–188
Okada Y, Minakami H, Tomomasa T, Kato M, Inoue Y, Kozawa K, Kimura H, Morikawa A (2004) Serum procalcitonin concentration in patients with Kawasaki disease. J Infect 48:199–205
Acknowledgements
We thank D. Baker (Department of Anesthesiology and Critical Care, CHU Necker-Enfants Malades, Paris, France) for reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hausfater, P., Hurtado, M., Pease, S. et al. Is procalcitonin a marker of critical illness in heatstroke?. Intensive Care Med 34, 1377–1383 (2008). https://doi.org/10.1007/s00134-008-1083-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-008-1083-y