Abstract
Summary
Osteoporosis (weak bones) is a disorder that has high morbidity, mortality, and healthcare utilization. Effective treatment is available for this disorder, but many patients choose not to start therapy. This is the first study showing an intervention that increases the initiation rates to medications for osteoporosis.
Introduction
One out of six patients prescribed an oral bisphosphonate does not initiate therapy, a phenomenon known as primary non-adherence. Reasons for bisphosphonate primary non-adherence have been identified, but not interventions that positively impact primary adherence rates. The purpose of this study is to determine the effectiveness of interactive voice response technology to improve oral bisphosphonate primary adherence.
Methods
This was a prospective, randomized controlled trial conducted in January–December 2014 at Kaiser Permanente Colorado, an integrated healthcare system. Adults with a new oral bisphosphonate prescription for osteoporosis or osteopenia which was not purchased within 14–20 days of being ordered were included. There were 127 and 118 patients in the intervention group and control groups, respectively. The intervention group received an interactive voice response phone call followed by a letter 1 week later if primary non-adherence continued, whereas the control group did not receive any outreach. The primary outcome was the proportion of patients who purchased their oral bisphosphonate within 25 days of randomization.
Results
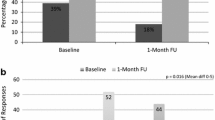
There were 62/127 (48.8 %) intervention patients and 36/118 (30.5 %) control patients who purchased their bisphosphonate prescription within 25 days of randomization (OR = 2.17, 95 % CI 1.29–3.67). When adjusted for age, sex, history of bone mineral density scan and fracture, the odds ratio for intervention versus control group was 2.3 (95 % CI 1.34–3.94).
Conclusion
An interactive voice response phone call and follow-up letter significantly improved primary adherence to oral bisphosphonate therapy. Such an intervention could be considered for improving primary adherence rates to other medication classes.



Similar content being viewed by others
References
National Osteoporosis Foundation (2013) What is osteoporosis? http://www.nof.org/articles/7. Accessed 9 Sep 2013
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348(9041):1535–1541
Andrade SE, Majumdar SR, Chan KA, Buist DS, Go AS, Goodman M, Smith DH, Platt R, Gurwitz JH (2003) Low frequency of treatment of osteoporosis among postmenopausal women following a fracture. Arch Intern Med 163:2052–2057
Yood RA, Mazar KM, Andrade SE, Emani S, Chan W, Kahler KH (2008) Patient decision to initiate therapy for osteoporosis: the influence of knowledge and beliefs. J Gen Intern Med 23(11):1815–1821
Gadkari AS, McHorney CA (2010) Medication nonfulfillment rates and reasons: narrative systematic review. Curr Med Res Opin 26:683–705
Hogan KN, Milchak JL, Heilmann RMF, Billups SJ, Delate T (2013) Evaluation of primary nonadherence to oral bisphosphonate therapy. J Am Geriatr Soc 61(11):2046–2047
Derose SF, Green K, Marrett E, Tunceli K, Cheetham TC, Chiu VY, Harrison TN, Reynolds K, Vansomphone SS, Scott RD (2013) Automated outreach to increase primary adherence to cholesterol-lowering medications. JAMA Intern Med 173(1):38–43
Sale JE, Gignac MA, Hawker G, Frankel L, Beaton D, Bogoch E, Elliot-Gibson V (2011) Decision to take osteoporosis medication in patients who have had a fracture and are ‘high’ risk for future fracture: a qualitative study. BMC Musculoskelet Disord 12:92
Schousboe JT, Dowd BE, Davison ML, Kane RL (2010) Association of medication attitudes with non-persistence and non-compliance with medication to prevent fractures. Osteoporos Int 21:1899–1909
Penning-van Beest FJA, Erkens JA, Olson M, Herings RMC (2008) Loss of treatment benefit due to low compliance with bisphosphonate therapy. Osteoporos Int 19:511–517
Fenerty SD, West C, Davis SA, Kaplan SG, Feldman SR (2012) The effect of reminder systems on patients’ adherence to treatment. Patient Prefer Adherence 6:127–135
Delmas PD, Vrijens B, Eastell R, Roux C, Pols HA, Ringe JD, Grauer A, Cahall D, Watts NB, Improving Measurements of Persistence on Actonel Treatment (IMPACT) Investigators (2007) Effect of monitoring bone turnover markers on persistence with risedronate treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab 92(4):1296–1304
Clowes JA, Peel NF, Eastell R (2004) The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab 89(3):1117–1123
Cramer JA, Amonkar MM, Hebborn A, Altman R (2005) Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin 21(9):1453–1460
Recker RR, Gallagher R, MacCosbe PE (2005) Effect of dosing frequency on bisphosphonate medication adherence in a large longitudinal cohort of women. Mayo Clin Proc 80(7):856–861
Karter AJ, Parker MM, Moffet HH, Ahmed AT, Schmittdiel JA, Selby JV (2009) New prescription medication gaps: a comprehensive measure of adherence to new prescriptions. Health Serv Res 44(5):1640–1661
Liberman JN, Hutchins DS, Popiel RG, Patel MH, Jan SA, Berger JE (2010) Determinants of primary nonadherence in asthma-controller and dyslipidemia pharmacotherapy. Am J Pharm Benefits 2(2):111–118
Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B (2014) The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res 29(11):2520–2526
Acknowledgments
This study was funded by Kaiser Permanente Colorado. The institution had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 144 kb)
Rights and permissions
About this article
Cite this article
Cizmic, A.D., Heilmann, R.M.F., Milchak, J.L. et al. Impact of interactive voice response technology on primary adherence to bisphosphonate therapy: a randomized controlled trial. Osteoporos Int 26, 2131–2136 (2015). https://doi.org/10.1007/s00198-015-3116-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3116-z