Abstract
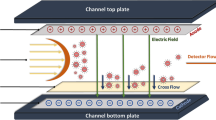
A method for determining the size of silver nanoparticles and their quantification by asymmetric flow field-flow fractionation coupled with inductively coupled plasma mass spectrometry (ICP-MS) is proposed and was tested in consumer products. Experimental conditions were studied in detail to avoid aggregation processes or alteration of the original size distributions. Additionally, losses from sorption processes onto the channel membrane were minimized for correct quantification of the nanoparticles. Mobile phase composition, injection/focusing, and fractionation conditions were evaluated in terms of their influence on both separation resolution and recovery. The ionic strength, pH, and the presence of ionic and nonionic surfactants had a strong influence on both separation and recovery of the nanoparticles. In general, better results were obtained under those conditions that favored charge repulsions with the membrane. Recovery values of 83 ± 8% and 93 ± 4% with respect to the content of silver nanoparticles were achieved for the consumer products studied. Silver nanoparticle standards were used for size calibration of the channel. The results were compared with those obtained by photon correlation spectroscopy and images taken by transmission electron microscopy. The quantification of silver nanoparticles was performed by direct injection of ionic silver standard solutions into the ICP-MS system, integration of the corresponding peaks, and interpolation of the fractogram area. A limit of detection of 5.6 μg L-1 silver, which corresponds to a number concentration of 1×1012 L-1 for nanoparticles of 10 nm, was achieved for an injection volume of 20 μL.





Similar content being viewed by others
References
Cumberland SA, Lead JR (2009) J Chromatogr A 1216:9099–9105
Shrivastava S, Bera T, Roy A, Singh G, Ramachandrarao P, Dash D (2007) Nanotechnology 18:225103
Benn T, Westerhoff P (2008) Environ Sci Technol 42:4133–4139
Andreescu S, Njagi J, Ispas C, Ravalli MT (2009) J Environ Monit 11:27–40
Domingos RF, Baalousha MA, Ju-Nam Y, Reid MM, Tufenkji N, Lead JR, Leppard GG, Wilkinson KJ (2009) Environ Sci Technol 43:7277–7284
Contado C, Pagnoni A (2008) Anal Chem 80:7594–7608
Howard AG (2010) J Environ Monit 12:135–142
Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, McLaughlin MJ, Lead JR (2008) Environ Toxicol Chem 27:1825–1851
Alvarez PJJ, Colvin V, Lead J, Stone V (2009) ACS Nano 3:1616–1619
Tiede K, Hassellöv M, Breitbarth E, Chaudhry Q, Boxall ABA (2009) J Chromatogr A 1216:503–509
Plathe KL, von der Kammer F, Hassellov M, Moore J, Murayama M, Hofmann T, Hochella MF (2009) Environ Chem 7:82–93
Hassellöv M, Lyven B, Haraldson C, Sirinawin W (1999) Anal Chem 71:3497–3502
Dubascoux S, Le Hecho I, Potin-Gautier M, Lespes G (2008) Talanta 77:60–65
Roda B, Zattoni A, Reschiglian P, Moon MH, Mirasoli M, Michelini E, Roda A (2009) Anal Chim Acta 635:132–143
Contado C, Dalpiaz A, Leo E, Zborowski M, Williams PS (2007) J Chromatogr A 1157:321–335
Benincasa MA, Mazzoni V (2007) J Liq Chromatogr Relat Technol 30:453–462
Rameshwar T, Samal S, Lee S, Kim S, Cho J, Kim IS (2006) J Nanosci Nanotechnol 6:2461–2467
Bouby M, Geckeis H, Geyer FW (2008) Anal Bioanal Chem 392:1447–1457
Sermsri W, Jarujamrus P, Shiowatana J, Siripinyanond A (2010) Anal Bioanal Chem 396:3079–3085
Poda AR, Bednar AJ, Kennedy AJ, Harmon A, Hull M, Mitrano DM, Ranville JF, Steevens J (2011) J Chromatogr A 1218:4219–4225
Schmidt B, Petersen JH, Koch CB, Plackett D, Johansen NR, Katiyar V, Larsen EH (2009) Food Addit Contam A 26:1619–1627
Ranville JF, Chittleborough DJ, Shanks F, Morrison RJS, Harris T, Doss F, Beckett RD (1999) Anal Chim Acta 381:315–329
Stolpe B, Hassellov M, Andersson K, Turner DR (2005) Anal Chim Acta 535:109–121
Thang NM, Geckeis H, Kim JI, Beck HP (2001) Colloids Surf A 181:289–301
Kvitek L, Panacek A, Soukupova J, Kolar M, Vecerova R, Prucek R, Holecova M, Zboril R (2008) J Phys Chem C 112:5825–5834
Christian P, Von der Kammer F, Baalousha M, Hofmann T (2008) Ecotoxicology 17:326–343
Susanto H, Ulbricht M (2009) Membr Sci 327:125–135
Ise N, Sogami IS (2005) Structure formation in solution: ionic polymers and colloidal particles. Springer, Berlin
Dubascoux S, Le Hecho I, Hassellöv M, Von Der Kammer F, Potin Gautier M, Lespes G (2010) J Anal At Spectrom 25:613–623
Gimbert LJ, Andrew KN, Haygarth PM, Worsfold PJ (2003) Trends Anal Chem 22:615–633
Wahlund KG, Giddings JC (1987) Anal Chem 59:1332–1339
Litzen A, Wahlund KG (1991) Anal Chem 63:1001–1007
Qureshi RN, Kok WTh (2010) LG/GC Eur 23:18–24
Laborda F, Jiménez-Lamana J, Bolea E, Castillo JR (2011) J Anal At Spectrom 26:1372–1379
Acknowledgements
This work was sponsored by the Spanish Ministry of Science and Innovation (project CTQ2009-14237-C02-01). The authors also thank Laboratorios Argenol S.L. for providing the Collargol samples, Gemma Cepria for the differential pulse anodic stripping voltammetry measurements, and Yolanda Rodas from the Institute of Nanoscience of Aragon for the PCS and zeta potential measurements. ICP-MS measurements were performed in the facilities of the Analytical Central Laboratory of the University of Zaragoza.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the special issue Plasma Spectrochemistry with Guest Editors Juan Castillo and Martín Resano.
Rights and permissions
About this article
Cite this article
Bolea, E., Jiménez-Lamana, J., Laborda, F. et al. Size characterization and quantification of silver nanoparticles by asymmetric flow field-flow fractionation coupled with inductively coupled plasma mass spectrometry. Anal Bioanal Chem 401, 2723–2732 (2011). https://doi.org/10.1007/s00216-011-5201-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-5201-2