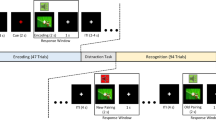
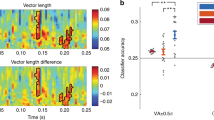
Abstract
The classification of repeating stimuli as either old or new is a general mechanism of everyday perception. However, the cortical mechanisms underlying this process are not fully understood. In general, mnemonic processes are thought to rely on changes in oscillatory brain activity across several frequencies as well as their interaction. Lower frequencies, mainly theta-band (3–7 Hz) and alpha-band (8–14 Hz) activity, are attributed to executive control and resource management, respectively; whereas recent studies revealed higher frequencies, e.g. gamma-band (> 25 Hz) activity, to reflect the activation of cortical object representations. Furthermore, low-frequency phase to high-frequency amplitude coupling (PAC) was recently found to coordinate the involved mnemonic networks. To further unravel the processes behind memorization of repeatedly presented stimuli, we applied a continuous item recognition task with up to five presentations per item (mean time between repetitions ~ 10 s) while recording high-density EEG. We examined spectral amplitude modulations as well as PAC. We observed theta amplitudes reaching a peak at second presentation, a reduction of alpha suppression after second presentation, decreased response time, as well as reduced theta–gamma PAC (3 to 7 to − 30 to 45 Hz) at frontal sites after third presentation. We conclude a shift from an explicit- to an implicit-like mnemonic processing, occurring around third presentation, with theta power to signify encoding of repetition-based episodic information and PAC as a neural correlate of the coordination of local neural networks.




Similar content being viewed by others
References
Axmacher N, Henseler MM, Jensen O, Weinreich I, Elger CE, Fell J (2010) Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc Natl Acad Sci 107(7):3228–3233
Bertrand O, Pantev C (1994) Stimulus frequency dependence of the transient oscillatory auditory evoked responses (40 Hz) studied by electric and magnetic recordings in human. In: Oscillatory event-related brain dynamics. Springer US, New York, pp 231–242
Bertrand O, Tallon-Baudry C (2000) Oscillatory gamma activity in humans: a possible role for object representation. Int J Psychophysiol 38(3):211–223
Brainard DH, Vision S (1997) The psychophysics toolbox. Spat Vis 10:433–436
Burgess AP, Ali L (2002) Functional connectivity of gamma EEG activity is modulated at low frequency during conscious recollection. Int J Psychophysiol 46(2):91–100
Canolty RT, Knight RT (2010) The functional role of cross-frequency coupling. Trends Cogn Sci 14(11):506–515
Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT (2006) High gamma power is phase-locked to theta oscillations in human neocortex. Science 313(5793):1626–1628
Chaumon M, Schwartz D, Tallon-Baudry C (2009) Unconscious learning versus visual perception: dissociable roles for gamma oscillations revealed in MEG. J Cogn Neurosci 21(12):2287–2299
Cowan N (1999) An embedded-processes model of working memory. Models of working memory. Mech Active Maint Exec Control 20:506
Cowan N (2008) What are the differences between long-term, short-term, and working memory? Progress Brain Res 169:323–338
Craddock M, Martinovic J, Müller MM (2016) Accounting for microsaccadic artifacts in the EEG using independent component analysis and beamforming. Psychophysiology 53(4):553–565
Curran T (1999) The electrophysiology of incidental and intentional retrieval: erp old/ new effects in lexical decision and recognition memory. Neuropsychologia 37(7):771–785
Daume J, Graetz S, Gruber T, Engel AK, Friese U (2017a) Cognitive control during audiovisual working memory engages frontotemporal theta-band interactions. Sci Rep 7(1):12585
Daume J, Gruber T, Engel AK, Friese U (2017b) Phase-amplitude coupling and long-range phase synchronization reveal frontotemporal interactions during visual working memory. J Neurosci 37(2):313–322
Delorme A, Makeig S (2004) EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134(1):9–21
Fries P, Nikolić D, Singer W (2007) The gamma cycle. Trends Neurosci 30(7):309–316
Friese U, Supp GG, Hipp JF, Engel AK, Gruber T (2012a) Oscillatory MEG gamma band activity dissociates perceptual and conceptual aspects of visual object processing: a combined repetition/conceptual priming study. Neuroimage 59(1):861–871
Friese U, Rahm B, Hassler U, Kaiser J, Gruber T (2012b) Repetition suppression and effects of familiarity on blood oxygenation level dependent signal and gamma-band activity. Neuroreport 23(13):757–761
Friese U, Köster M, Hassler U, Martens U, Trujillo-Barreto N, Gruber T (2013) Successful memory encoding is associated with increased cross-frequency coupling between frontal theta and posterior gamma oscillations in human scalp-recorded EEG. Neuroimage 66:642–647
Gevins A, Smith ME, McEvoy L, Yu D (1997) High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cereb Cortex (New York, NY: 1991) 7(4):374–385
Graf P, Schacter DL (1985) Implicit and explicit memory for new associations in normal andamnesic subjects. J Exp Psychol Learn Mem Cogn 11(3):501
Gruber T, Müller MM (2006) Oscillatory brain activity in the human EEG during indirect and direct memory tasks. Brain Res 1097(1):194–204
Gruber T, Tsivilis D, Montaldi D, Müller MM (2004) Induced gamma band responses: an early marker of memory encoding and retrieval. Neuroreport 15(11):1837–1841
Gruber T, Tsivilis D, Giabbiconi CM, Müller MM (2008) Induced electroencephalogram oscillations during source memory: familiarity is reflected in the gamma band, recollection in the theta band. J Cogn Neurosci 20(6):1043–1053
Hanslmayr S, Staudigl T (2014) How brain oscillations form memories—a processing based perspective on oscillatory subsequent memory effects. Neuroimage 85:648–655
Hanslmayr S, Spitzer B, Bäuml KH (2008) Brain oscillations dissociate between semantic and nonsemantic encoding of episodic memories. Cereb Cortex 19(7):1631–1640
Hassler U, Trujillo-Barreto N, Gruber T (2011) Induced gamma band responses in human EEG after the control of miniature saccadic artifacts. Neuroimage 57(4):1411–1421
Hassler U, Friese U, Martens U, Trujillo-Barreto N, Gruber T (2013) Repetition priming effects dissociate between miniature eye movements and induced gamma-band responses in the human electroencephalogram. Eur J Neurosci 38(3):2425–2433
Headley DB, Paré D (2017) Common oscillatory mechanisms across multiple memory systems. npj Sci Learn 2(1):1
Heusser AC, Poeppel D, Ezzyat Y, Davachi L (2016) Episodic sequence memory is supported by a theta–gamma phase code. Nat Neurosci 19(10):1374
Jacobs J, Hwang G, Curran T, Kahana MJ (2006) EEG oscillations and recognition memory: theta correlates of memory retrieval and decision making. Neuroimage 32(2):978–987
Jensen O, Colgin LL (2007) Cross-frequency coupling between neuronal oscillations. Trends Cogn Sci 11(7):267–269
Jensen O, Mazaheri A (2010) Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci 4:186
Jensen O, Kaiser J, Lachaux JP (2007) Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci 30(7):317–324
Johnson DR, Gronlund SD (2009) Individuals lower in working memory capacity are particularly vulnerable to anxiety’s disruptive effect on performance. Anxiety Stress Coping 22(2):201–213
Johnson JD, Muftuler LT, Rugg MD (2008) Multiple repetitions reveal functionally and anatomically distinct patterns of hippocampal activity during continuous recognition memory. Hippocampus 18(10):975–980
Jokisch D, Jensen O (2007) Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. J Neurosci 27(12):3244–3251
Jutras MJ, Buffalo EA (2010) Synchronous neural activity and memory formation. Curr Opin Neurobiol 20(2):150–155
Kleiner M, Brainard D, Pelli D, Ingling A, Murray R, Broussard C (2007) What’s new in Psychtoolbox-3. Perception 36(14):1
Klimesch W, Doppelmayr M, Schwaiger J, Winkler T, Gruber W (2000) Theta oscillations and the ERP old/new effect: independent phenomena? Clin Neurophysiol 111(5):781–793
Klimesch W, Sauseng P, Hanslmayr S (2007) EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev 53(1):63–88
Köster M, Friese U, Schöne B, Trujillo-Barreto N, Gruber T (2014) Theta–gamma coupling during episodic retrieval in the human EEG. Brain Res 1577:57–68
Kramer MA, Tort AB, Kopell NJ (2008) Sharp edge artifacts and spurious coupling in EEG frequency comodulation measures. J Neurosci Methods 170(2):352–357
LaRocque JJ, Lewis-Peacock JA, Drysdale AT, Oberauer K, Postle BR (2013) Decoding attended information in short-term memory: an EEG study. J Cogn Neurosci 25(1):127–142
Lewis-Peacock JA, Drysdale AT, Oberauer K, Postle BR (2012) Neural evidence for a distinction between short-term memory and the focus of attention. J Cogn Neurosci 24(1):61–79
Lisman JE, Jensen O (2013) The theta-gamma neural code. Neuron 77(6):1002–1016
Mormann F, Fell J, Axmacher N, Weber B, Lehnertz K, Elger CE, Fernández G (2005) Phase/amplitude reset and theta–gamma interaction in the human medial temporal lobe during a continuous word recognition memory task. Hippocampus 15(7):890–900
Nolan H, Whelan R, Reilly RB (2010) FASTER: fully automated statistical thresholding for EEG artifact rejection. J Neurosci Methods 192(1):152–162
Nyhus E, Curran T (2010) Functional role of gamma and theta oscillations in episodic memory. Neurosci Biobehav Rev 34(7):1023–1035
Osipova D, Takashima A, Oostenveld R, Fernández G, Maris E, Jensen O (2006) Theta and gamma oscillations predict encoding and retrieval of declarative memory. J Neurosci 26(28):7523–7531
Roux F, Uhlhaas PJ (2014) Working memory and neural oscillations: alpha–gamma versus theta–gamma codes for distinct WM information? Trends Cogn Sci 18(1):16–25
Ruchkin DS, Grafman J, Cameron K, Berndt RS (2003) Working memory retention systems: a state of activated long-term memory. Behav Brain Sci 26(6):709–728
Rugg MD, Curran T (2007) Event-related potentials and recognition memory. Trends Cogn Sci 11(6):251–257
Sarnthein J, Petsche H, Rappelsberger P, Shaw GL, Von Stein A (1998) Synchronization between prefrontal and posterior association cortex during human working memory. Proc Natl Acad Sci 95(12):7092–7096
Sauseng P, Klimesch W, Doppelmayr M, Hanslmayr S, Schabus M, Gruber WR (2004) Theta coupling in the human electroencephalogram during a working memory task. Neurosci Lett 354(2):123–126
Sauseng P, Klimesch W, Schabus M, Doppelmayr M (2005) Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. Int J Psychophysiol 57(2):97–103
Sauseng P, Griesmayr B, Freunberger R, Klimesch W (2010) Control mechanisms in working memory: a possible function of EEG theta oscillations. Neurosci Biobehav Rev 34(7):1015–1022
Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR (2003) Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci 23(34):10809–10814
Sederberg PB, Gauthier LV, Terushkin V, Miller JF, Barnathan JA, Kahana MJ (2006) Oscillatory correlates of the primacy effect in episodic memory. NeuroImage 32(3):1422–1431
Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsáki G (2008) Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron 60(4):683–697
Tallon-Baudry C, Bertrand O (1999) Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci 3(4):151–162
Tort AB, Komorowski R, Eichenbaum H, Kopell N (2010) Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol 104(2):1195–1210
Tulving E, Schacter DL (1990) Priming and human memory systems. Science 247(4940):301–306
Vidal JR, Chaumon M, O’Regan JK, Tallon-Baudry C (2006) Visual grouping and the focusing of attention induce gamma-band oscillations at different frequencies in human magnetoencephalogram signals. J Cogn Neurosci 18(11):1850–1862
Wiggs CL, Martin A (1998) Properties and mechanisms of perceptual priming. Curr Opin Neurobiol 8(2):227–233
Wixted JT (2007) Dual-process theory and signal-detection theory of recognition memory. Psychol Rev 114(1):152
Xu Y (2017) Reevaluating the sensory account of visual working memory storage. Trends Cogn Sci 21(10):794–815
Yassa MA, Stark CE (2008) Multiple signals of recognition memory in the medial temporal lobe. Hippocampus 18(9):945–954
Yonelinas AP (2002) The nature of recollection and familiarity: a review of 30 years of research. J Mem Lang 46(3):441–517
Acknowledgements
This work was supported by grants from the German Research Foundation (GR2684/5-1). We thank Moritz Köster for helpful methodological discussions, and Simeon Platte, Sophia Sylvester and Marlene Wessels for assistance in data recording.
Funding
This work was supported by grants from the German Research Foundation (GR2684/5-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Osnabrück University and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was reviewed and approved by the local ethics committee of Osnabrück University.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Graetz, S., Daume, J., Friese, U. et al. Alterations in oscillatory cortical activity indicate changes in mnemonic processing during continuous item recognition. Exp Brain Res 237, 573–583 (2019). https://doi.org/10.1007/s00221-018-5439-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-018-5439-4