Abstract
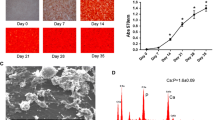
Biosilica is a natural polymer, synthesized by the poriferan enzyme silicatein from monomeric silicate substrates. Biosilica stimulates mineralizing activity and gene expression of SaOS-2 cells. To study its effect on the formation of hydroxyapatite (HA), SaOS-2 cells were grown on different silicatein/biosilica-modified substrates (bone slices, Ca–P-coated coverslips, glass coverslips). Growth on these substrates induced the formation of HA nodules, organized in longitudinal arrays or spherical spots. Nodules of sizes above 1 μm were composed of irregularly arranged HA prism-like nanorods, formed by aggregates of three to eight SaOS-2 cells. Moreover, growth on silicatein/biosilica-modified substrates elicited increased [3H]dT incorporation into DNA, indicative of enhanced cell proliferation. Consequently, an in vitro-based bioassay was established to determine the ratio between [3H]dT incorporation and HA formation. This ratio was significantly higher for cells that grew on silicatein/biosilica-modified substrates than for cells on Ca–P-coated coverslips or plain glass slips. Hence, we propose that this ratio of in vitro-determined parameters reflects the osteogenic effect of different substrates on bone-forming cells. Finally, qRT-PCR analyses demonstrated that growth of SaOS-2 cells on a silicatein/biosilica matrix upregulated BMP2 (bone morphogenetic protein 2, inducer of bone formation) expression. In contrast, TRAP (tartrate-resistant acid phosphatase, modulator of bone resorption) expression remained unaffected. We conclude that biosilica shows pronounced osteogenicity in vitro, qualifying this material for studies of bone replacement also in vivo.








Similar content being viewed by others
References
Carlisle EM (1970) Silicon: a possible factor in bone calcification. Science 167:279–280
Schwarz K, Milne DB (1972) Growth promoting effects of silicon in rats. Nature 239:333–334
Kinrade SD, Del Nin JW, Schach AS (1999) Stable five- and six-coordinated silicate anions in aqueous solution. Science 285:1542–1545
Kinrade SD, Balec RJ, Schach AS, Wang J, Knight CTG (2004) The structure of aqueous pentaoxo silicon complexes with cis-1,2-dihydroxycyclopentane and furanoidic vicinal cis-diols. Dalton Trans 21:3241–3243
Jugdaohsingh R (2007) Silicon and bone health. J Nutr Health Aging 11:99–110
Adler AJ, Berlyne GM (1986) Silicon metabolism II. Renal handling in chronic renal failure patients. Nephron 44:36–39
Schwarz K (1973) A bound form of silicon in glycosaminoglycans and polyuronides. Proc Natl Acad Sci USA 70:1608–1612
Carlisle EM (1976) In vivo requirement for silicon in articular cartilage and connective tissue formation in the chick. J Nutr 106:478–484
Carlisle EM (1981) Silicon in bone formation. In: Simpson TL, Volcani BE (eds) Silicon and siliceous structures in biological systems, vol 4. Springer Verlag, New York, pp 69–94
Hott M, de Pollak C, Modrowski D, Marie PJ (1993) Short-term effects of organic silicon on trabecular bone in mature ovariectomized rats. Calcif Tissue Int 53:174–179
Sandford F (2003) Physical and chemical analysis of the siliceous skeleton in six sponges of two groups (Demospongiae and Hexactinellida). Microsc Res Tech 62:336–355
Uriz MJ (2006) Mineral spiculogenesis in sponges. Can J Zool 84:322–356
Cha JN, Shimizu K, Zhou Y, Christianssen SC, Chmelka BF, Stucky GD, Morse DE (1999) Silicatein filaments and subunits from a marine sponge direct the polymerization of silica and silicones in vitro. Proc Natl Acad Sci USA 96:361–365
Krasko A, Batel R, Schröder HC, Müller IM, Müller WEG (2000) Expression of silicatein and collagen genes in the marine sponge Suberites domuncula is controlled by silicate and myotrophin. Eur J Biochem 267:4878–4887
Iler RK (1979) The chemistry of silica. Plenum Press, New York
Shanklin DR, Smalley DL (1998) The immunopathology of siliconosis: history, clinical presentation, and the chemistry of silicon and silicone. Immunol Res 18:124–173
Cooper C, Dennison E (1998) Do silicone breast implants cause connective tissue disease? BMJ 316:403–404
Schröder HC, Borejko A, Krasko A, Reiber A, Schwertner H, Müller WEG (2005) Mineralization of SaOS-2 cells on enzymatically (Silicatein) modified bioactive osteoblast-stimulating surfaces. J Biomed Mater Res B 75:387–392
Müller WEG, Boreiko A, Wang XH, Krasko A, Geurtsen W, Custódio MR, Winkler T, Lukić-Bilela L, Link T, Schröder HC (2007) Morphogenetic activity of silica and biosilica on the expression of genes, controlling biomineralization using SaOS-2 cells. Calcif Tissue Int 81:382–393
Adkisson HD, Strauss-Schoenberger J, Gillis M, Wilkins R, Jackson M, Hruska KA (2000) Rapid quantitative bioassay of osteoinduction. J Orthop Res 18:503–511
Albrektsson T, Johansson C (2001) Osteoinduction, osteoconduction and osseointegration. Eur Spine J 10:S96–S101
Hausser HJ, Brenner RE (2005) Phenotypic instability of SaOS-2 cells in long-term culture. Biochem Biophys Res Commun 333:216–222
Kelly SE, Di Benedetto A, Greco A, Howard CM, Sollars VE, Primerano DA, Valluri JV, Claudio PP (2010) Rapid selection and proliferation of CD133+ cells from cancer cell lines: chemotherapeutic implications. PLoS ONE 5:e10035. doi:10.1371/journal.pone.0010035
Tanaka H, Nagai E, Murata H, Tsubone T, Shirakura Y, Sugiyama T, Taguchi T, Kawai S (2001) Involvement of bone morphogenic protein-2 (BMP-2) in the pathological ossification process of the spinal ligament. Rheumatology 40:1163–1168
Fromigue O, Hay E, Modrowski D, Bouvet S, Jacquel A, Auberge P, Marie PJ (2006) RhoA GTPase inactivation by statins induces osteosarcoma cell apoptosis by inhibiting p42/p44-MAPKs-Bcl-2 signaling independently of BMP-2 and cell differentiation. Cell Death Differ 13:1845–1856
Li Q, Kannan A, Wang W, Demayo FJ, Taylor RN, Bagchi MK, Bagchi IC (2007) Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J Biol Chem 282:31725–31732
Nickel J, Dreyer MK, Kirsch T, Sebald W (2001) The crystal structure of the BMP-2:BMPR-IA complex and the generation of BMP-2 antagonists. J Bone Joint Surg 83:S7–S14
Matsuzaki K, Katayama K, Takahashi Y, Nakamura I, Udagawa N, Tsurukai T, Nishinakamura R, Toyama Y, Yabe Y, Hori M, Takahashi N, Suda T (1999) Human osteoclast-like cells are formed from peripheral blood mononuclear cells in a coculture with SaOS-2 cells transfected with the parathyroid hormone (PTH)/PTH-related protein receptor gene. Endocrinology 140:925–932
Hollberg K, Nordahl J, Hultenby K, Mengarelli-Widholm S, Andersson G, Reinholt FP (2005) Polarization and secretion of cathepsin K precede tartarate-resistant acid phosphatase secretion to the ruffled border area during the activation of matrix-resorbing clasts. J Bone Miner Metab 23:441–449
Oddie GW, Schenk G, Angel NZ, Walsh N, Guddat LW, de Jersey J, Cassady AI, Hamilton SE, Hume DA (2000) Structure, function, and regulation of tartrate-resistant acid phosphatase. Bone 27:575–584
Fogh J, Fogh JM, Orfeo T (1977) One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst 59:221–226
Zhou Z, Han JY, Xi CX, Xie JX, Feng X, Wang CY, Mei L, Xiong WC (2008) HMGB1 regulates RANKL-induced osteoclastogenesis in a manner dependent on RAGE. J Bone Miner Res 23:1084–1096
Jiang X, Ye M, Jiang X, Liu G, Feng S, Cui Zou H (2007) Method development of efficient protein extraction in bone tissue for proteome analysis. J Proteome Res 6:2287–2294
Röder W, Müller H, Merz H, Müller WEG (1992) HIV-infection in human bone. J Bone Joint Surg Br 74:179–180
Natalio F, Link T, Müller WEG, Schröder HC, Cui FZ, Wang XH, Wiens M (2010) Bioengineering of the silica-polymerizing enzyme silicatein-α for a targeted application to hydroxyapatite. Acta Biomater 6:3720–3728
Müller WEG, Rothenberger M, Boreiko A, Tremel W, Reiber A, Schröder HC (2005) Formation of siliceous spicules in the marine demosponge Suberites domuncula. Cell Tissue Res 321:285–297
Gregory CA, Gunn WG, Peister A, Prockop DJ (2004) An alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem 329:77–84
Shimo T, Matsumura S, Ibaragi S, Isowa S, Kishimoto K, Mese H, Nishiyama A, Sasaki A (2007) Specific inhibitor of MEK-mediated cross-talk between ERK and p38 MAPK during differentiation of human osteosarcoma cells. J Cell Commun Signal 1:103–111
Endo A, Yamada M, Kataoka S, Sano T, Inagi Y, Miyaki A (2010) Direct observation of surface structure of mesoporous silica with low acceleration voltage FE-SEM. Colloids Surf A 357:11–16
Müller WEG, Wang X, Schröder HC, Korzhev M, Grebenjuk VA, Markl JS, Jochum KP, Pisignano D, Wiens M (2010) A cryptochrome-based photosensory system in the siliceous sponge Suberites domuncula (Demospongiae). FEBS J 277:1182–1201
Schwartz Z, Fisher M, Lohmann CH, Simon BJ, Boyan BD (2009) Osteoprotegerin (OPG) production by cells in the osteoblast lineage is regulated by pulsed electromagnetic fields in cultures grown on calcium phosphate substrates. Ann Biomed Eng 37:437–444
Borsje MA, Ren Y, de Haan-Visser HW, Kuijer R (2010) Comparison of low-intensity pulsed ultrasound and pulsed electromagnetic field treatments on OPG and RANKL expression in human osteoblast-like cells. Angle Orthod 80:498–503
Kochanowska I, Chaberek S, Wojtowicz A, Marczyński B, Włodarski K, Dytko M, Ostrowski K (2007) Expression of genes for bone morphogenetic proteins BMP-2, BMP-4 and BMP-6 in various parts of the human skeleton. BMC Musculoskelet Disord 8:128
Bitenc LD, Komadina R, Preželj J, Ostanek B, Trošt Z, Marc J (2007) Expression of bone resorption genes in osteoarthritis and in osteoporosis. J Bone Miner Metab 25:219–225
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2002–2007
Sachs L (1984) Angewandte Statistik. Springer, Berlin
Morgan EF, Barnes GL, Einhorn TA (2008) The bone organ system: form and function. In: Marcus R, Feldman D, Nelson DA, Rosen CJ (eds) Osteoporosis, 3rd edn. Elsevier, San Diego, pp 3–25
Müller WEG (2006) The stem cell concept in sponges (Porifera): metazoan traits. Semin Cell Dev Biol 17:481–491
Kaandorp JA, Blom JG, Verhoef J, Filatov M, Postma M, Müller WEG (2008) Modelling genetic regulation of growth and form in a branching sponge. Proc Biol Sci 275:2569–2575
Schröder HC, Boreiko A, Korzhev M, Tahir MN, Tremel W, Eckert C, Ushijima H, Müller IM, Müller WEG (2006) Co-expression and functional interaction of silicatein with galectin: matrix-guided formation of siliceous spicules in the marine demosponge Suberites domuncula. J Biol Chem 281:12001–12009
Hay E, Lemonnier J, Fromigue O, Guenou H, Pierre JM (2004) Bone morphogenetic protein receptor IB signaling mediates apoptosis independently of differentiation in osteoblastic cells. J Biol Chem 279:1650–1658
Postiglione L, DiDomenico G, Montagnani S, Di Spigna G, Salzano S, Castaldo C, Ramaglia L, Sbordone L, Rossi G (2003) Granulocyte macrophage colony-stimulating factor (GM-CSF) induces the osteoblastic differentiation of the human osteosarcoma cell line SaOS-2. Calcif Tissue Int 72:85–97
Nissinen L, Pirila L, Heino J (1997) Bone morphogenetic protein-2 is a regulator of cell adhesion. Exp Cell Res 230:377–385
McCullough KA, Waits CA, Garimella R, Tague SE, Sipe JB, Anderson HC (2007) Immunohistochemical localization of bone morphogenetic proteins (BMPs) 2, 4, 6, and 7 during induced heterotopic bone formation. J Orthop Res 25:465–472
Schmidbaur H, Bach I, Wilkinson DL, Müller G (1989) Preparation and crystal structures of magnesium, strontium, and barium l-glutamate hydrates. Chem Ber 122:1433–1438
Chen H, Clarkson BH, Sun K, Mansfield JF (2005) Self-assembly of synthetic hydroxyapatite nanorods into an enamel prism-like structure. J Colloid Interface Sci 288:97–103
Hernandez-Pando R, Bornstein QL, Aguilar Leon D, Orozco EH, Madrigal VK, Martinez Cordero E (2000) Inflammatory cytokine production by immunological and foreign body multinucleated giant cells. Immunology 100:352–358
Kii I, Amizuka N, Shimomura J, Saga Y, Kudo A (2004) Cell–cell interaction mediated by cadherin-11 directly regulates the differentiation of mesenchymal cells into the cells of the osteo-lineage and the chondro-lineage. J Bone Miner Res 19:1840–1849
Ghanaati S, Orth C, Barbeck M, Willershausen I, Thimm BW, Booms P, Stübinger S, Landes C, Sader RA, Kirkpatrick CJ (2010) Histological and histomorphometrical analysis of a silica matrix embedded nanocrystalline hydroxyapatite bone substitute using the subcutaneous implantation model in Wistar rats. Biomed Mater 5:035005
Spencer GJ, Utting JC, Etheridge SL, Arnett TR, Genever PG (2006) Wnt signalling in osteoblasts regulates expression of the receptor activator of NFκB ligand and inhibits osteoclastogenesis in vitro. J Cell Sci 119:1283–1296
Eliseev RA, Schwarz EM, Zuscik MJ, O’Keefe Regis J, Drissi H, Rosier RN (2006) Smad7 mediates inhibition of Saos2 osteosarcoma cell differentiation by NFκnB. Exp Cell Res 312:40–50
Katz JM, Nataraj C, Jaw R, Deigl E, Bursac P (2008) Demineralized bone matrix as an osteoinductive biomaterial and in vitro predictors of its biological potential. J Biomed Mater Res B 89:127–134
Cao JJ, Wronski TJ, Iwaniec U, Phleger L, Kurimoto P, Boudignon B, Halloran BP (2006) Aging increases stromal/osteoblastic cell-induced osteoclastogenesis and alters the osteoclast precursor pool in the mouse. J Bone Miner Res 20:1659–1668
Wilson TJ, Nannuru KC, Futakuchi M, Sadanandam A, Singh RK (2008) Cathepsin G enhances mammary tumor-induced osteolysis by generating soluble receptor activator of nuclear factor-κB ligand. Cancer Res 68:5803–5811
Tahir MN, Théato P, Müller WEG, Schröder HC, Janshoff A, Zhang J, Huth J, Tremel W (2004) Monitoring the formation of biosilica catalysed by histidin-tagged silicatein. ChemComm 24:2848–2849
Jin H, Heller DA, Sharma R, Strano MS (2009) Size-dependent cellular uptake and expulsion of single-walled carbon nanotubes: single particle tracking and a generic uptake model for nanoparticles. Nano 3:149–158
Thamatrakoln K, Alverson AJ, Hildebrand M (2006) Comparative sequence analysis of diatom silicon transporters: towards a mechanistic model of silicon transport. J Phycol 42:822–834
Schröder HC, Krasko A, Le Pennec G, Adell T, Hassanein H, Müller IM, Müller WEG (2003) Silicase, an enzyme which degrades biogenous amorphous silica: contribution to the metabolism of silica deposition in the demosponge Suberites domuncula. Prog Mol Subcell Biol 33:249–268
Wetzel P, Hasse A, Papadopoulos S, Voipio J, Kaila K, Gros G (2001) Extracellular carbonic anhydrase activity facilitates lactic acid transport in rat skeletal muscle fibres. J Physiol 531:743–756
Gao BB, Clermont A, Rook S, Fonda SJ, Srinivasan VJ, Wojtkowski M, Fujimoto JG, Avery RL, Arrigg PG, Bursell SE, Aiello LP, Feener E (2007) Extracellular carbonic anhydrase mediates hemorrhagic retinal and cerebral vascular permeability through prekallikrein activation. Nat Med 13:181–188
Schröder HC, Natalio F, Shukoor I, Tremel W, Schloßmacher U, Wang XH, Müller WEG (2007) Apposition of silica lamellae during growth of spicules in the demosponge Suberites domuncula: biological/biochemical studies and chemical/biomimetical confirmation. J Struct Biol 159:325–334
Gröger C, Sumper M, Brunner E (2007) Silicon uptake and metabolism of the marine diatom Thalassiosira pseudonana: solid-state 29Si NMR and fluorescence microscopic studies. J Struct Biol 161:55–63
Wiens M, Mangoni A, D’Esposito M, Fattorusso E, Korchagina N, Schröder HC, Grebenjuk VA, Krasko A, Batel R, Müller IM, Müller WEG (2003) The molecular basis for the evolution of the metazoan bodyplan: extracellular matrix-mediated morphogenesis in marine demosponges. J Mol Evol 57:S60–S75
De Martino A, Amato A, Bowler C (2009) Mitosis in diatoms: rediscovering an old model for cell division. BioEssays 31:874–884
Hanks SK, Quinn AM (1991) Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods Enzymol 200:38–62
Wiens M, Wang X, Natalio F, Schröder HC, Schloßmacher U, Wang S, Korzhev M, Geurtsen W, Müller WEG (2010) Bioinspired fabrication of biosilica-based bone substitution materials. Adv Eng Mater. doi:10.1002/adem.200980043
Acknowledgments
This work was supported by grants from the German Bundesministerium für Bildung und Forschung (project Center of Excellence BIOTECmarin), the Deutsche Forschungsgemeinschaft (Schr 277/10-1), the International Human Frontier Science Program, the European Commission (project 244967-Mem-S [Bottom-up design and fabrication of industrial bio-inorganic nanoporous membranes with novel functionalities based on principles of protein self-assembly and biomineralization]), the Johannes Gutenberg-University Research Center for Complex Matter (COMATT), and the International S & T Cooperation Program of China (grant 2008DFA00980).
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have stated that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wiens, M., Wang, X., Schloßmacher, U. et al. Osteogenic Potential of Biosilica on Human Osteoblast-Like (SaOS-2) Cells. Calcif Tissue Int 87, 513–524 (2010). https://doi.org/10.1007/s00223-010-9408-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-010-9408-6