Abstract
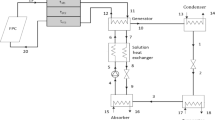
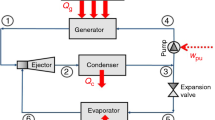
The present work provides a detailed thermodynamic analysis of a 10 kW solar absorption refrigeration system using ammonia-water mixtures as a working medium. This analysis includes both first law and second law of thermodynamics. The coefficient of performance (COP), exergetic coefficient of performance (ECOP) and the exergy losses (ΔE) through each component of the system at different operating conditions are obtained. The minimum and maximum values of COP and ECOP were found to be at 110 and 200°C generator temperatures respectively. About 40% of the system exergy losses were found to be in the generator. The maximum exergy losses in the absorber occur at generator temperature of 130°C for all evaporator temperatures. A computer simulation model is developed to carry out the calculations and to obtain the results of the present study.


















Similar content being viewed by others
Abbreviations
- COP:
-
Coefficient of performance
- C p :
-
Specific heat (kJ/kg K)
- E :
-
Exergy (kW)
- ECOP:
-
Exergetic coefficient of performance
- f :
-
Circulation ratio
- g :
-
Specific Gibbs energy (kJ/kg)
- G :
-
Total Gibbs energy (kJ)
- h :
-
Specific enthalpy (kJ/kg)
- I :
-
Availability destruction
- KE:
-
Kinetic energy (kJ)
- m :
-
Mass (kg)
- n :
-
Number of moles
- P :
-
Pressure (kPa)
- PE:
-
Potential energy (kJ)
- Q :
-
Heat energy (kW)
- REV:
-
Refrigerant expansion valve
- \( \overset{\lower0.5em\hbox{$\smash{\scriptscriptstyle\frown}$}}{R} \) :
-
Gas constant (kJ/kmol K)
- s :
-
Specific entropy (kJ/kg K)
- S :
-
Total entropy (kJ/K)
- SEV:
-
Solution expansion valve
- t :
-
Time (s)
- T :
-
Temperature (°C)
- U :
-
Internal energy (kJ)
- v :
-
Specific volume (m3/kg)
- W :
-
Work (kJ)
- x :
-
Liquid ammonia mass fraction
- y :
-
Vapor ammonia mass fraction
- Δ:
-
Difference in any quantity
- ∂ :
-
Partial differential
- μ :
-
Chemical potential
- ε :
-
Effectiveness
- A:
-
Ammonia
- abs:
-
Absorber
- cond:
-
Condenser
- cv:
-
Control volume
- e:
-
Exit
- eq:
-
Equilibrium
- evap:
-
Evaporator
- gen:
-
Generator
- i:
-
Inlet
- in:
-
Input
- out:
-
Output
- o:
-
Reference state
- p:
-
Constant pressure
- r:
-
reduced properties
- ref:
-
Refrigerant
- regen:
-
Regenerator
- REV:
-
Refrigerant expansion valve
- SEV:
-
Solution expansion valve
- W:
-
Water
- E:
-
Excess property
- L:
-
Liquid
- M:
-
Mixture
- o:
-
Rate of flow of property
- tot:
-
Total value
- V:
-
Vapor
- ^:
-
Properties per unit kmol
References
Bula A, Diane H, Luis FN, Lesme AC (2000) Thermodynamics simulation of solar absorption refrigeration systems, generator heat exchanger. AMSE2000, France
Alvares SG, Trepp Ch (1987) Simulation of a solar driven aqua-ammonia absorption refrigeration system. Int J T Refrig 10:40–48
Zigler F, Alefeld G (1987) Coefficient of performance of multistage absorption cycles. Int J Refrig 10:285–295
Karakas A, Egrican N, Uygur S (1990) Second-law analysis of solar absorption-cooling cycles using lithium bromide/water and ammonia/water as working fluids. Appl Energy 37:169–187
Wang W, Wang RZ, Xu YX, Wu JY, Gui YB (1998) Investigation on adsorption refrigeration with a single adsorbent bed. Int J Energy Res 22:1157–1163
Balamura V, Ibrahim O, Barnett S (2000) Simulation of ternary ammonia–water–salt absorption refrigeration cycles. Int J Refrig 23:31–42
Morejon C (2001) Thermodynamics for the study of the heat and mass transfer for absorption refigration cycles with water-ammonia as fluid of work. International conference on computational heat and mass transfer, October 22–26, Brazil
Ataer O, Gogus Y (1991) Comparative study of irreversibilities in an aqua-ammonia absorption systems. Int J Refrig 14:86–92
Sikorask R, Maczek K (2000) Exergy losses of two stage absorption refrigeration system calculations and charts”. IIF-IIR, Commission B1, B2, and E2. Purdue University, USA, pp 497–504
ASHRAE (1997) ASHRAE fundamental handbook. American Society of Heating, Refrigeration and Air-Conditioning Engineers, New York
Aphornratana S, Eames IW (1995) Thermodynamic analysis of absorption refrigeration cycles using the second law of thermodynamics method. Int J Refrig 18(4):244–252
Cekvenik B, Satzger P, Ziegler F, Poredos A (1999) High efficient sorption cycles using CaO/H2O and LiBr/H2O for gas cooling’. 3rd ASME/RAES renewable and advanced energy systems for 21st century conference, April 11–14, Hawaii
Gordon JM, Ng KC, Chua HT (1999) Simple thermodynamic diagrams for real refrigeration systems. J Appl Phys 85(2):641–646
Alvares SF, Trepp Ch (1987) Simulation of a solar driven aqua-ammonia absorption refrigeration system. Int J Refrig 10:40–48
Kuehn TH, Ramsey JW, Threlkeld JL (1998) Thermal environmental engineering, 3rd edn. Prentice-Hall, Englewood Cliffs
Machielsen CHM (1997) Solar powered refrigeration by means of ammonia/water absorption cycles. Energy Systems and Environmental Research Institute, pp 115–127
Amano Y, Suzuki T, Hashizume T, Akaiba M, Tanzawa Y, Usui A (2000) A hybrid power generation and refrigeration cycle with ammonia–water mixture. In: Proceedings of 2000 international joint power generation conference, Miami, 23–26 July, ASME, pp 1–6
Patek J, Klomfar J (1995) Simple function for fast calculation of selected thermodynamic properties of ammonia–water system. Int J Refrig 18(4):228–234
Rukes B, Dooley R (2001) Guideline on the IAPWS formulation 2001 for the thermodynamic properties of ammonia–water mixtures. IAPWS September, USA
Hammad M, Zurigat Y (1997) Performance of second generation solar cooling units. Energy Systems and Environmental Research Institute, pp 129–143
Landauro J, Watson F, Holland F (1983) Experimental study of the operation characteristics of a water–lithium bromide absorption cooler. Chem Eng Res Des 61:362–370
Ma WB, Deng SM (1996) Theoretical analysis of low-temperature hot source driven two-stage LiBr/H2O absorption refrigeration system. Int J Refrig 19(2):141–146
Zigler B, Trep PC (1984) Equation of state for ammonia–water mixtures. Revue international due froid 7:101–106
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abu-Ein, S.Q., Fayyad, S.M., Momani, W. et al. Performance analysis of solar powered absorption refrigeration system. Heat Mass Transfer 46, 137–145 (2009). https://doi.org/10.1007/s00231-009-0538-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-009-0538-1