Abstract
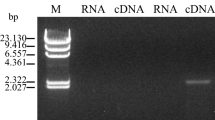
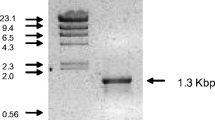
The lactonase gene of Fusarium oxysporum was expressed in Aspergillus oryzae for optical resolution of dl-pantoyl lactone. When the chromosomal gene encoding the full-length form of the lactonase, which has its own NH2-terminal signal peptide, was introduced in the host cells, the resulting transformant produced an enzyme of 46,600 Da, which corresponded to the wild-type enzyme. In contrast, A. oryzae transformed with the cDNA coding the mature enzyme produced a protein of 41,300 Da. Deglycosylation analysis with an endoglycosidase revealed that the difference in molecular mass arose from the different sugar contents of the recombinant enzymes. The mycelia of the transformant were used as a catalyst for asymmetric hydrolysis of dl-pantoyl lactone. The initial velocity of the asymmetric hydrolysis reaction catalyzed by the transformant was estimated to be 30 times higher than that by F. oxysporum. When the mycelia of the transformant were incubated with a 20% dl-pantoyl lactone solution for 4 h, 49.9% of the racemic mixture was converted to d-pantoic acid (>95% ee).




Similar content being viewed by others
References
Archer DB, Jeenes DJ, Mackenzie DA, Brightwell G, Lambert N, Lowe G, Radford SE, Dobson CM (1990) Hen egg white lysozyme expressed in, and secreted from, Aspergillus niger is correctly processed and folded. Biotechnology 8:741–745
Bradford MM (1966) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Gomi K, Iimura Y, Hara S (1987) Integrative transformation of Aspergillus oryzae with a plasmid containing the Aspergillus nidulans argB gene. Agric Biol Chem 51:2549–2555
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Biol Chem 166:557–580
Honda K, Kataoka M, Shimizu S (2002) Functional analyses and application of microbial lactonohydrolases. Biotechnol Bioprocess Eng 7:130–137
Juge N, Svensson B, Williamson G (1998) Secretion, purification, and characterization of barley alpha-amylase produced by heterologous gene expression in Aspergillus niger. Appl Microbiol Biotechnol 49:385–392
Kataoka M, Shimizu K, Sakamoto K, Yamada H, Shimizu S (1995a) Optical resolution of racemic pantolactone with a novel fungal enzyme, lactonohydrolase. Appl Microbiol Biotechnol 43:974–977
Kataoka M, Shimizu K, Sakamoto K, Yamada H, Shimizu S (1995b) Lactonohydrolase-catalyzed optical resolution of pantoyl lactone: selection of a potent enzyme producer and optimization of culture and reaction conditions for practical resolution. Appl Microbiol Biotechnol 44:333–338
Kataoka M, Rohani LPS, Yamamoto K, Wada M, Kawabata H, Kita K, Yanase H, Shimizu S (1997) Enzymatic production of ethyl (R)-4-chloro-3-hydroxybutanoate: asymmetric reduction of ethyl 4-chloro-3-oxobutanoate by Escherichia coli transformant expressing the aldehyde reductase gene from yeast. Appl Microbiol Biotechnol 48:699–703
Kataoka M, Yamamoto K, Kawabata H, Wada M, Kita K, Yanase H, Shimizu S (1999) Stereoselective reduction of ethyl 4-chloro-3-oxobutanoate by Escherichia coli transformant cells coexpressing the aldehyde reductase and glucose dehydrogenase genes. Appl Microbiol Biotechnol 51:486–490
King J, Laemmli UK (1971) Polypeptides of the tail fibers of bacteriophage T4. J Mol Biol 62:465–477
Kizaki N, Yasohara Y, Hasegawa J, Wada M, Kataoka M, Shimizu S (2001) Synthesis of optical pure ethyl (S)-4-chloro-3-hydroxybutanoate by Escherichia coli transformant cells coexpressing the carbonyl reductase and glucose dehydrogenase genes. Appl Microbiol Biotechnol 55:590–595
Malardier L, Daboussi MJ, Julien J, Roussel F, Scazzocchio C, Brygoo Y (1989) Cloning of the nitrate reductase gene (niaD) of Aspergillus nidulans and its use for transformation of Fusarium oxysporum. Gene 78:147–156
Minetoki T, Kumagai C, Gomi K, Kitamoto K, Takahashi K (1998) Improvement of promoter activity by the introduction of multiple copies of the conserved region III sequence, involved in the efficient expression of Aspergillus oryzae amylase-encoding genes. Appl Microbiol Biotechnol 50:459–467
Shimizu S, Kataoka M, Shimizu K, Hirakata M, Sakamoto K, Yamada H (1992) Purification and characterization of a novel lactonohydrolase, catalyzing the hydrolysis of aldonate lactones and aromatic lactones, from Fusarium oxysporum. Eur J Biochem 209:383–390
Shimizu S, Kataoka M, Sakamoto K (2003) Preparation of lactonase and its application. Jpn patent 2003-371750
Toda T, Sano M, Honda M, Rimoldi OJ, Yang Y, Yamamoto M, Takase K, Hirozumi K, Kitamoto K, Minetoki T, Gomi K, Machida M (2001) Deletion analysis of the enolase gene (enoA) promoter from the filamentous fungus Aspergillus oryzae. Curr Genet 40:260–267
Yasohara Y, Kizaki N, Hasegawa J, Wada M, Kataoka M, Shimizu S (2001) Stereoselective reduction of alkyl 3-oxobutanoate by carbonyl reductase from Candida magnoliae. Tetrahedron Asymmetry 12:1713–1718
Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research, numbers 1729 (to K.H.) and 14360054 (to M.K.), from the Japan Society for the Promotion of Science. This work was also supported in part by the “COE for Microbial-Process Development Pioneering Future Production System”, J-3 (to S.S.), of the COE Program of the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Honda, K., Tsuboi, H., Minetoki, T. et al. Expression of the Fusarium oxysporum lactonase gene in Aspergillus oryzae: molecular properties of the recombinant enzyme and its application. Appl Microbiol Biotechnol 66, 520–526 (2005). https://doi.org/10.1007/s00253-004-1758-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-004-1758-4