Abstract
To utilize fermentative bacteria for producing the alternative fuel hydrogen, we performed successive rounds of P1 transduction from the Keio Escherichia coli K-12 library to introduce multiple, stable mutations into a single bacterium to direct the metabolic flux toward hydrogen production. E. coli cells convert glucose to various organic acids (such as succinate, pyruvate, lactate, formate, and acetate) to synthesize energy and hydrogen from formate by the formate hydrogen-lyase (FHL) system that consists of hydrogenase 3 and formate dehydrogenase-H. We altered the regulation of FHL by inactivating the repressor encoded by hycA and by overexpressing the activator encoded by fhlA, removed hydrogen uptake activity by deleting hyaB (hydrogenase 1) and hybC (hydrogenase 2), redirected glucose metabolism to formate by using the fdnG, fdoG, narG, focA, focB, poxB, and aceE mutations, and inactivated the succinate and lactate synthesis pathways by deleting frdC and ldhA, respectively. The best of the metabolically engineered strains, BW25113 hyaB hybC hycA fdoG frdC ldhA aceE, increased hydrogen production 4.6-fold from glucose and increased the hydrogen yield twofold from 0.65 to 1.3 mol H2/mol glucose (maximum, 2 mol H2/mol glucose).
Similar content being viewed by others
Introduction
Hydrogen is the most abundant element in the universe (Dunn 2002), is renewable, efficient, and clean (Hansel and Lindblad 1998), and is utilized for fuel cells in portable electronics, power plants, and the internal combustion engine (Dunn 2002). It is estimated that the global energy system will shift from fossil fuels to hydrogen and methane (Dunn 2002). Most of the hydrogen now produced globally is by the process of steam reforming and the water–gas shift reaction (Yi and Harrison 2005), or as a by-product of petroleum refining and chemical production (Das and Veziroğlu 2001). Use of biological methods of hydrogen production should significantly reduce energy costs, as these processes do not require extensive heating (or extensive electricity as in electrolysis plants; Das and Veziroğlu 2001). Biological methods depend on hydrogenases, which catalyze the reaction 2H+ + 2e− ↔ H2 (g) (Evans and Pickett 2003). Hydrogen may be produced through either photosynthetic or fermentative processes, but fermentative hydrogen production is more efficient than photosynthetic production (Yoshida et al. 2005).
Escherichia coli is used in this study for hydrogen production, as it is easy to manipulate genetically, and it is the best-characterized bacterium (Blattner et al. 1997). For example, the glucose glycolytic pathway to phosphoenolpyruvate, pyruvate, acetate, ethanol, and formate via bacterial fermentation is well established (Bagramyan and Trchounian 2003), and P1 phage transduction allows one to easily introduce mutations into E. coli cells. Previously, we (Maeda et al. 2007b) used the isogenic E. coli K-12 KEIO collection of the Genome Analysis Project in Japan (Baba et al. 2006), which contains all non-lethal deletion mutations (3985 genes), to introduce as many as six mutations in a single E. coli strain for directing cell metabolism from formate to hydrogen without diminishing cell growth. The simple technique consisted of removing the kanamycin antibiotic resistance marker (kanR) after each round of P1 transduction by using the flanking flippase (FLP) recognition target sequences with FLP recombinase (Datsenko and Wanner 2000).
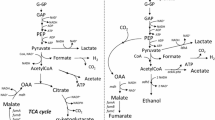
E. coli produces hydrogen from formate by the formate hydrogen lyase system (FHL) that consists of hydrogenase 3 (encoded by hycABCDEFGHI; Bagramyan and Trchounian 2003) and formate dehydrogenase-H (encoded by fdhF; Axley et al. 1990); these enzymes catalyze the reaction \({\text{HCOO}}^ - + {\text{H}}_2 {\text{O}} \leftrightarrow {\text{H}}_2 + {\text{HCO}}_{\text{3}} ^ - \) (Woods 1936; Fig. 1) and are probably used to help regulate internal pH (Böck and Sawers 1996). FHL activity is repressed by the hycA gene product (Bagramyan and Trchounian 2003) and activated by the fhlA gene product (Schlensog et al. 1994); hence, the FHL may be manipulated to increase hydrogen by overexpression of fhlA (Yoshida et al. 2005) and deletion of hycA (Penfold et al. 2003; Yoshida et al. 2005). The evolved hydrogen from the FHL is consumed by E. coli hydrogenase 1 (hyaB encodes the large subunit; Forzi and Sawers 2007) and hydrogenase 2 (hybC encodes the large subunit; Forzi and Sawers 2007; Fig. 1). In E. coli, there are also two additional formate dehydrogenases encoded by fdnG (α-subunit of formate dehydrogenase-N) and fdoG (α-subunit of formate dehydrogenase-O) that serve to consume formate (Rossmann et al. 1991). Also, focA (Suppmann and Sawers 1994) and focB (Andrews et al. 1997) encode proteins that export formate, and nitrate reductase A (α-subunit encoded by narG) consumes formate by converting nitrate into nitrite by using electrons produced from formate by formate dehydrogenase-N (Bertero et al. 2003). Hence, by deleting hyaB, hybC, fdnG, fdoG, focAB, and narG, hydrogen production should be enhanced, and we have found that a quintuple mutant (BW25113 hyaB hybC hycA fdoG/pCA24N-FhlA) increases hydrogen production from formate by over two orders of magnitude (Maeda et al. 2007b). In addition, pyruvate dehydrogenase (encoded by aceE) and pyruvate oxidase (encoded by poxB) consume pyruvate produced from glucose (Abdel-Hamid et al. 2001; Angelides et al. 1979; Fig. 1), so inactivating these genes may be useful for enhancing hydrogen production by preventing pyruvate consumption. Also, the succinate-producing pathway (phosphoenolpyruvate to succinate) and lactate-producing pathway (pyruvate to lactate) may be inactivated to direct glucose metabolism toward hydrogen (Fig. 1); therefore, deletion of fumarate reductase (frdC) and lactate dehydrogenase (ldhA) increases hydrogen production from glucose (Yoshida et al. 2006).
Schematic of fermentative hydrogen production in E. coli. Cells metabolize glucose into phosphoenolpyruvate, pyruvate, and formate. Phosphoenolpyruvate is converted to succinate by fumarate reductase (FrdC), and pyruvate is converted to either lactate by lactate dehydrogenase (LdhA), to carbon dioxide (CO2) and acetate by pyruvate oxidase (PoxB), to carbon dioxide by pyruvate dehydrogenase (AceE), or to formate by pyruvate formate lyase (PFL). Hydrogen is produced from formate by the formate hydrogen lyase (FHL) system consisting of hydrogenase 3 (Hyd 3) and formate dehydrogenase-H (FDHH); the FHL is activated by FhlA that is regulated by Fnr and repressed by HycA. Evolved hydrogen is consumed through the hydrogen uptake activity of hydrogenase 1 (Hyd 1) and hydrogenase 2 (Hyd 2). Formate is exported by FocA and/or FocB and is metabolized by formate dehydrogenase-N (FDHN; FdnG), which is linked with nitrate reductase A (NarG) and formate dehydrogenase-O (FDHO; FdoG). HypABCDEF are maturation proteins for hydrogenases 1, 2, and 3
Because it may be more practical to produce hydrogen from glucose (Kraemer and Bagley 2007) rather than to add or overproduce formate, in this study, we create one septuple mutant (BW25113 hyaB hybC hycA fdoG frdC ldhA aceE) that produces 4.6-fold more hydrogen than the wild-type strain and that enhances the yield of hydrogen twofold as a result of manipulating the pathway mutations hyaB, hybC, hycA, fhlA, focA, focB, narG, fdoG, fdnG, frdC, ldhA, poxB, and aceE. This is the first report of strains harboring these seven mutations for converting glucose to hydrogen (previously, we reported on an E. coli strain harboring the hyaB hybC hycA, fdoG and fhlA mutations for converting formate to hydrogen (Maeda et al. 2007b)), and this is the first investigation of the importance of the poxB and aceE mutations for hydrogen production.
Materials and methods
Bacterial strains, growth rates, and total protein
Strains are shown in Table 1. E. coli cells were initially streaked from −80°C glycerol stocks on Luria–Bertani (LB) agar plates (Sambrook et al. 1989) containing 100 μg/ml kanamycin (for those with chromosomal kanamycin resistance markers) and 30 μg/ml chloramphenicol (for those containing pCA24N-based plasmids), and incubated at 37°C. After growth on LB agar plates, a fresh single colony was cultured at 37°C with shaking at 250 rpm (New Brunswick Scientific Co., Edison, NJ, USA) in LB medium (Sambrook et al. 1989) or in modified complex glucose medium (Rachman et al. 1997) in which 0.4 mg/l (NH4)6Mo7O24 was added; 100 μg/ml kanamycin or 30 μg/ml chloramphenicol were also added where appropriate. Wild-type E. coli K-12 BW25113 was obtained from the Yale University CGSC Stock Center, and its isogenic deletion mutants (Keio collection) were obtained from the Genome Analysis Project in Japan (Baba et al. 2006). Plasmids based on pCA24N (Kitagawa et al. 2005) were electroporated into hydrogen-overproducing E. coli strains (Table 1). Aerobic cell growth was measured using turbidity at 600 nm from 0.05 to 0.7, and total protein for E. coli was 0.22 mg/OD per ml (Protein assay kit, Sigma Diagnostics, St. Louis, MO, USA).
Multiple chromosomal mutations
Repeated rounds of P1 transduction (Silhavy et al. 1984) were performed to knockout specific genes by selecting for the kanamycin-resistance gene that is transferred along with each chromosomal deletion that are available from the Keio collection (Baba et al. 2006). Each Keio deletion mutant is designed with the ability to eliminate the kanamycin-resistance selection marker by expressing the FLP recombinase protein from pCP20 (Cherepanov and Wackernagel 1995), as each kanamycin resistance gene is flanked by a FLP recognition target that is excised by FLP recombinase. Hence, plasmid pCP20 (Cherepanov and Wackernagel 1995) was used as described previously (Datsenko and Wanner 2000) to eliminate the kanamycin resistance gene from each isogenic BW25113 mutant allele that was transferred to the chromosome via each P1 transduction so that multiple mutations could be introduced into a single strain.
Hydrogen closed vial assay
Overnight, aerobic cultures (25 ml) were used to inoculate 75 ml of the modified complex glucose medium (111 mM glucose) in 250-ml shake flasks, and these cultures were sparged for 5 min with nitrogen, sealed, and incubated anaerobically at 37oC for 6 h. After 6 h, the cultures were poured anaerobically into a 250-mL centrifuge tubes in an anaerobic glove box, and centrifuged (7,350×g) for 10 min at 4oC. The supernatant was decanted in the glove box, 20 ml of modified complex medium without glucose was added, and then the cells were suspended to a turbidity of 2.5 at 600 nm. Sealed crimp-top vials (27 ml) were sparged for 5 min with nitrogen, and 9 ml of the cell suspension and 1 ml of 1 M glucose were added to the bottles that were incubated at 37oC with shaking for 30 min to 17 h. The amount of hydrogen generated in the head space of the recombinant system was measured using a 50-μl aliquot by gas chromatography (GC) using a 6890N gas chromatograph as described previously (Maeda et al. 2007c).
Hydrogen-low partial pressure assay
Cells (30 ml) were prepared as above for the closed system, sparged, sealed in crimp-top vials (60 ml), 100 mM glucose was added, then the hydrogen gas was allowed to leave the headspace through a needle in the septum via tubing that directed the gas through 1 M NaOH (to remove carbon dioxide; Klibanov et al. 1982), and into an inverted graduated cylinder that was used to measure the volume of the gas (Maeda et al. 2007b). The vials were incubated at 37°C with stirring for 15 min, and hydrogen was assayed with a GC. As a negative control, cell suspensions (20 ml) without glucose were also used. Glucose concentrations in complex glucose media were measured using the HK assay (Sigma). For yield calculations, the vials were incubated for 16 h.
Results
Our strategy for metabolic engineering of E. coli for enhanced hydrogen production from glucose via formate was sixfold (Fig. 1) and based on our initial success of using some of these mutations for increasing the yield of hydrogen from formate using strain BW25113 hyaB hybC hycA fdoG/pCA24N-FhlA (Maeda et al. 2007b); note that all the original mutations had to be reevaluated, as they were originally assayed for their effect on producing hydrogen starting from formate, and two new mutations were evaluated here (poxB and aceE). We (1) prevented hydrogen consumption by inactivating hydrogenase 1 (HyaB, large subunit) and hydrogenase 2 (HybC, large subunit), (2) inactivated the FHL repressor HycA, (3) overexpressed the FHL activator FhlA (FhlA binds directly to the intergenic region between the hyc and hyp operons and between the hycA and hycB genes; Schlensog et al. 1994), (4) eliminated the formate exporters FocA and its homolog FocB (Andrews et al. 1997; Suppmann and Sawers 1994), (5) prevented formate consumption by formate dehydrogenase-N (FdnG, α-subunit) coupled with nitrate reductase A (NarG, α-subunit; Rossmann et al. 1991) and dehydrogenase-O (FdoG, α-subunit; Rossmann et al. 1991), and (6) altered glucose metabolism to efficiently synthesize formate from glucose by preventing lactate and succinate formation, as well as pyruvate consumption. E. coli cells metabolize glucose into formate via phosphoenolpyruvate and pyruvate by the glycolytic system (Bagramyan and Trchounian 2003); phosphoenolpyruvate may also be converted into succinate by fumarate reductase (FrdC; Iverson et al. 1999), pyruvate may be converted into lactate by lactate dehydrogenase (LdhA; Sode et al. 1999), and pyruvate may be consumed by pyruvate dehydrogenase (AceE; Angelides et al. 1979) and pyruvate oxidase (PoxB; Abdel-Hamid et al. 2001; Fig. 1). Therefore, deleting frdC, ldhA, aceE, and poxB should enhance hydrogen production by increasing formate production.
Another goal was to introduce mutations that did not make the cell less viable so specific growth rates were quantified after each mutation was added. Cell viability was not significantly affected for all strains (46 strains) except the two septuple mutants with the aceE mutations (hyaB hybC hycA fdoG frdC ldhA aceE and hyaB hybC hycA fdnG frdC ldhA aceE) that had a 3.6-fold reduced aerobic specific growth rate compared to the wild type strain in LB medium (Table 2). In addition, the specific growth rate of BW25113 hyaB hybC hycA fdoG frdC ldhA aceE was 2.7 times lower than that of wild-type cells in complex glucose medium (1.6 ± 0.1 for BW25113 vs 0.59 ± 0.02 1/h for BW25113 hyaB hybC hycA fdoG frdC ldhA aceE); however, there was no difference in the amount of cellular protein after overnight growth for the low partial pressure/closed hydrogen assay experiments between the wild-type strain and the hyaB hybC hycA fdoG frdC ldhA aceE strain (data not shown). These results are primarily in contrast to other approaches in which cell viability has been reduced (Penfold et al. 2006).
To decrease hydrogen uptake activity in E. coli, the genes encoding the large subunits of hydrogenase 1 (hyaB) and hydrogenase 2 (hybC) were deleted because the active site of catalysis is located within each large subunit for these [NiFe]-hydrogenases (Forzi and Sawers 2007). As expected, the double mutant (hyaB hybC) showed a significant decrease in hydrogen uptake activity (Maeda et al. 2007a) that led to a 1.4-fold increase in hydrogen production compared to the wild-type strain in complex glucose medium after 30 min (Table 2); however, there was only a slight change in hydrogen production rates for each single mutation (hyaB or hybC). Also, adding the hycA mutation to the hyaB hybC double mutant did not show a significant increase in hydrogen production from glucose (Table 2), although BW25113 hyaB hybC hycA produced 1.5-fold more hydrogen from formate compared to cells defective in both hydrogenase 1 (hyaB) and hydrogenase 2 (hybC; Maeda et al. 2007b).
Formate, which is the substrate for producing hydrogen in E. coli, is depleted by two non-hydrogen-producing pathways: (1) excretion by the formate transporter FocA (Suppmann and Sawers 1994) and its homolog FocB (Andrews et al. 1997), and (2) degradation by formate dehydrogenase-N (coupled with nitrate reductase A) and degradation by formate dehydrogenase-O, which convert formate to ATP (Wang and Gunsalus 2003). Hence, formate transport, formate dehydrogenase-N/nitrate reductase A activity, and formate dehydrogenase-O activity may be deleted to enhance hydrogen production. Based on this strategy, five quadruple mutants (hyaB hybC hycA focA, hyaB hybC hycA focB, hyaB hybC hycA narG, hyaB hybC hycA fdnG, and hyaB hybC hycA fdoG) were constructed by introducing a focA, focB, narG, fdnG, and fdoG mutation to the triple mutant (hyaB hybC hycA), and then hydrogen production was assayed. The addition of the focB and narG mutations to the hyaB hybC hycA mutant increased hydrogen production 1.6-fold compared to the wild-type strain in complex glucose medium after 30 min (Table 2). Also, hydrogen production in BW25113 hyaB hybC hycA fdoG was increased 1.7-fold compared to that in the wild-type cells (Table 2). The focA and fdnG mutation were not effective for producing more hydrogen in the hyaB hybC hycA background.
To further test the combination of the focA, focB, narG, fdnG, and fdoG mutations, seven quintuple strains (hyaB hybC hycA focA focB, hyaB hybC hycA focA narG, hyaB hybC hycA focB narG, hyaB hybC hycA focB fdnG, hyaB hybC hycA focB fdoG, hyaB hybC hycA fdnG fdoG, and hyaB hybC hycA fdoG focA) and two sextuple strains (hyaB hybC hycA focA focB narG and hyaB hybC hycA focB fdnG fdoG) were constructed, and hydrogen production was assayed. Three quintuple mutants (hyaB hybC hycA fdnG fdoG, hyaB hybC hycA fdoG focA, and hyaB hybC hycA focA focB) produced 1.5–1.7 times more hydrogen than the wild-type strain; hydrogen production in two quintuple mutants BW25113 hyaB hybC hycA focA narG and hyaB hybC hycA focB narG was the same level with that in BW25113 hyaB hybC hycA. On the other hand, two quintuple mutants (hyaB hybC hycA focB fdnG and hyaB hybC hycA focB fdoG) and two sextuple strains (hyaB hybC hycA focA focB narG and hyaB hybC hycA focB fdnG fdoG) had lower hydrogen production activity than the wild-type cells.
To test the effect of deleting the succinate-producing pathway (frdC) and the lactate-producing pathway (ldhA), two quadruple mutants (hyaB hybC hycA ldhA and hyaB hybC hycA frdC), seven quintuple mutants (hyaB hybC hycA ldhA frdC, hyaB hybC hycA fdoG ldhA, hyaB hybC hycA fdoG frdC, hyaB hybC hycA focB ldhA, hyaB hybC hycA focB frdC, hyaB hybC hycA narG ldhA, and hyaB hybC hycA narG frdC), three sextuple mutants (hyaB hybC hycA fdnG fdoG ldhA, hyaB hybC hycA fdoG ldhA frdC, and hyaB hybC hycA fdnG ldhA frdC), and one septuple mutant (hyaB hybC hycA fdoG fdnG ldhA frdC) were constructed, and hydrogen production was assayed. One quintuple (BW25113 hyaB hybC hycA frdC ldhA) and two sextuple mutants (BW25113 hyaB hybC hycA fdoG ldhA frdC and hyaB hybC hycA fdnG ldhA frdC) produced twofold more hydrogen than the wild-type strain after 30 min in complex glucose medium (Table 2). Also, hydrogen production in all strains harboring the ldhA mutation was increased by 20–50% compared to that in the wild-type cells after 17 h in complex glucose medium (Table 2). One septuple mutant (hyaB hybC hycA fdoG fdnG ldhA frdC) showed lower hydrogen production than two sextuple mutants (hyaB hybC hycA fdoG ldhA frdC and hyaB hybC hycA fdnG ldhA frdC) that have high hydrogen production potential (Table 2).
Previously, we found that expressing the FhlA protein (FHL activator) led to a ninefold increase in hydrogen production in medium containing formate (BW25113/pCA24N-FhlA vs BW25113; Maeda et al. 2007b). Hence, to boost hydrogen productivity further, plasmid pCA24N-FhlA was added to the metabolically engineered strains, and hydrogen production was assayed (Table 2). Unexpectedly, the expression of fhlA did not lead to a significant increase of hydrogen production from the modified complex-glucose medium in BW25113, BW25113 hyaB hybC hycA, BW25113 hyaB hybC hycA fdoG, and BW25113 hyaB hybC hycA fdoG ldhA frdC in the closed hydrogen assay. Also, overexpressing FhlA by adding isopropylthiogalactoside (IPTG; 0.01 to 1 mM) led to a significant decrease in hydrogen production; hydrogen production with 1 mM IPTG was threefold less than that without IPTG (data not shown).
To investigate whether pyruvate consumption by the PoxB and AceE pathways (Fig. 1) is significant for hydrogen production, four septuple mutants (BW25113 hyaB hybC hycA fdoG frdC ldhA aceE, BW25113 hyaB hybC hycA fdoG frdC ldhA poxB, BW25113 hyaB hybC hycA fdnG frdC ldhA aceE, and BW25113 hyaB hybC hycA fdnG frdC ldhA poxB) were constructed, and then hydrogen production was assayed. Two septuple strains with the aceE mutation (BW25113 hyaB hybC hycA fdoG frdC ldhA aceE and BW25113 hyaB hybC hycA fdnG frdC ldhA aceE) had a slight increase of hydrogen production (8 to 12%) compared to BW25113 hyaB hybC hycA fdoG frdC ldhA or BW25113 hyaB hybC hycA fdnG frdC ldhA; hydrogen production in these two strains was 2.2 times higher than that in wild-type strain (Table 2).
With BW25113 hyaB hybC hycA fdoG frdC aceE, we also tested whether the enhanced hydrogen production was from the added glucose. As expected, this strain produced hydrogen only slightly from complex medium that lacked glucose (4.4% of that from complex glucose medium). This indicates that hydrogen from complex glucose is derived from glucose.
Because the accumulation of hydrogen in the headspace in the closed system reduces hydrogen production (Kraemer and Bagley 2007), hydrogen production for the nine best strains was measured using an anaerobic system that maintained low hydrogen headspace pressure, and the results are shown in Table 3. BW25113 hyaB hybC hycA fdoG frdC ldhA aceE produced 4.6-fold more hydrogen than the wild-type strain, whereas BW25113 hyaB hybC hycA fdnG frdC ldhA, BW25113 hyaB hybC hycA fdnG frdC ldhA aceE, and BW25113 hyaB hybC hycA fdoG frdC ldhA had 4.1- to 4.3-fold higher hydrogen production. Similarly, BW25113 hyaB hybC hycA frdC, BW25113 hyaB hybC hycA ldhA, and BW25113 hyaB hybC hycA frdC ldhA synthesized 2.9- to 3.3-fold more hydrogen relative to the wild-type strain (Table 3). As a negative control, BW25113 hyaB hybC hycE, which lacks an active hydrogenase 3, showed negligible hydrogen production (8.8-fold less) than that of the wild-type cells for both the low partial pressure (Table 3), as well as the closed hydrogen assays (Table 2). Also, BW25113 hyaB hybC hycA fdoG ldhA frdC with pCA24N or pCA24N-FhlA produced up to 4.8-fold higher hydrogen than BW25113/pCA24N, although the over expression of FhlA protein did not lead to a significant increase of hydrogen production.
Along with hydrogen production, hydrogen yields are important. For BW25113 hyaB hybC hycA frdC ldhA, BW25113 hyaB hybC hycA fdnG frdC ldhA, BW25113 hyaB hybC hycA fdnG frdC ldhA aceE, BW25113 hyaB hybC hycA fdnG frdC ldhA poxB, BW25113 hyaB hybC hycA fdoG frdC ldhA, and BW25113 hyaB hybC hycA fdoG frdC ldhA aceE, the hydrogen yield increased by twofold compared to that in BW25113 wild-type cells. In addition, BW25113 hyaB hybC hycA fdoG frdC ldhA with pCA24N and pCA24N-FhlA had 2.5-fold higher hydrogen yields than BW25113/pCA24N. Also, the yield of BW25113 hyaB hybC hycA frdC and BW25113 hyaB hybC hycA ldhA increased 1.4 to 1.8-fold compared to BW25113 hyaB hybC hycA (Table 3), indicating that these two mutations (frdC and ldhA) are effective for enhancing hydrogen yields from glucose. Deleting aceE in BW25113 hyaB hybC hycA fdoG frdC ldhA (i.e., BW25113 hyaB hybC hycA fdoG frdC ldhA aceE) had a slight increase (up to 7%) in hydrogen yield compared to that in BW25113 hyaB hybC hycA fdoG frdC ldhA (Table 3). Assaying glucose in complex glucose medium demonstrated clearly that the septuple strain consumed over 97% of glucose after 16 h.
Discussion
In this work, we show that a fermentative E. coli strain with seven mutations, BW25113 hyaB hybC hycA fdoG frdC ldhA aceE, produces 4.6-fold more hydrogen than the wild-type strain (~32 μmol/h per mg protein vs 7 μmol/h per mg protein) as a result of inactivating hydrogen consumption by hydrogenase 1 (hyaB) and hydrogenase 2 (hybC), activation of FHL by deleting the FHL repressor (hycA), inactivation of formate dehydrogenase-O (fdoG) to prevent formate consumption, inactivation of the succinate synthesis (frdC) and lactate synthesis (ldhA) pathways, and inactivation of pyruvate dehydrogenase (aceE) to prevent pyruvate consumption. Also, the hydrogen yield with BW25113 hyaB hybC hycA fdoG frdC ldhA aceE by the strain increased twofold (∼1.32 vs 0.7 mol H2/mol glucose).
We used the E. coli Keio collection in this study to introduce as many as seven mutations into a single strain. Thus, the use of this library is a breakthrough in that it has been difficult to make strains with multiple mutations using other methods that depend on different selection makers for each gene inactivated (Lee et al. 2005; Yoshida et al. 2006). This method is general and simple (repetition of resistance-gene elimination and P1 transduction) and may be used to engineer E. coli for many applications where multiple chromosomal genes must be eliminated.
Previously, three groups have enhanced hydrogen production in E. coli. Inactivation of the FHL repressor (HycA) and overexpression of the FHL activator (FhlA) led to a 2.8-fold increase of hydrogen production from formate (Yoshida et al. 2005). Deleting the twin-arginine translocation system for transporting proteins into the periplasm resulted in twofold higher hydrogen production from glucose by indirectly inactivating hydrogenase 1, hydrogenase 2, formate dehydrogenase-N, and formate dehydrogenase-O; however, this mutation led to a significant decrease in cell viability (Penfold et al. 2006). Also, deletions of lactate dehydrogenase (ldhA) and fumarate reductase (frdBC) resulted in only a 1.4-fold increase in hydrogen production compared to the wild-type strain from glucose (Yoshida et al. 2006). In comparison, our metabolically engineered E. coli cells have as much as 4.6-fold greater hydrogen production, and the method remains robust, as it is still possible to introduce further mutations to enhance hydrogen production. Because of the ease of its genetic manipulation, E. coli may also be a better model than other hydrogen-producing strains such as Citrobacter sp. Y19 (Oh et al. 2003), Rhodopseudomonas palustris JA1 (Archana et al. 2003), Rhodopseudomonas palustris P4 (Jung et al. 1999), and Klebsiella oxytoca HP1 (Minnan et al. 2005) that have high maximum hydrogen activity (up to 65 μmol/mg per h).
The deletion of succinate-producing pathway (frdC) and lactate-producing pathway (ldhA) in the hyaB hybC hycA background led to a threefold higher increase of hydrogen production rate and a twofold higher hydrogen yield compared to the wild-type strain (Table 3); these results are consistent with the results described previously (Sode et al. 1999; Yoshida et al. 2006). Because the two quadruple mutants (BW25113 hyaB hybC hycA frdC and BW25113 hyaB hybC hycA ldhA) increased hydrogen production 1.5- and 1.4-fold, respectively, vs BW25113 hyaB hybC hycA (Table 3) and resulted in a 1.1- or 1.5-fold higher hydrogen yield relative to BW25113 hyaB hybC hycA, both the frdC and ldhA mutations are important for hydrogen production from glucose, but the ldhA mutation is more effective than the frdC mutation for increasing the hydrogen yield (Table 3).
It has been reported that the deficiency of formate dehydrogenase-N leads to an accumulation of intracellular formate and activation of the FHL pathway (Suppmann and Sawers 1994); hence, mutating fdnG should be effective for enhancing hydrogen production. As expected, the deletion of fdnG was significant as seen by comparing hydrogen production between BW25113 hyaB hybC hycA frdC ldhA vs BW25113 hyaB hybC hycA fdnG frdC ldhA (Table 3); the additional fdnG deletion led to a 45% increase in the hydrogen production rate. Similarly, deleting fdoG also increased hydrogen production by about 45% (BW25113 hyaB hybC hycA frdC ldhA vs BW25113 hyaB hybC hycA fdoG frdC ldhA, Table 3); however, the effect was not as large as the effect for growth on formate where there was a 2.2-fold increase in hydrogen production (BW25113 hyaB hybC hycA vs BW25113 hyaB hybC hycA fdoG in a closed system assay; Maeda et al. 2007b). On the other hand, deleing both formate dehydrogenase-N and formate dehydrogenase-O led to a significant decrease in the hydrogen production rate (Tables 2 and 3), although these mutations did not influence hydrogen yield. These results show that either active formate dehydrogenase-N or formate dehydrogenase-O is essential for producing hydrogen from glucose, whereas increasing hydrogen production from formate requires inactivation of formate dehydrogenase-O (Maeda et al. 2007b).
Because our metabolically engineered E. coli strains had a 1.3 mol H2/mol glucose of hydrogen yield instead of the theoretical hydrogen yield for facultative anaerobes of 2 mol H2/mol glucose (Yoshida et al. 2006), the E. coli cells metabolize glucose by pathways other than those remaining to make formate. For example, E. coli cells have three lactate dehydrogenases (ldhA, dld, and lldD) and two of them are membrane-bound flavoproteins linked with the respiratory chain (Mat-Jan et al. 1989); hence, these other two lactate dehyrogenases may prevent the cell from producing even more hydrogen.
The deletion of pyruvate oxidase (poxB) in the BW25113 hyaB hybC hycA fdoG frdC ldhA and BW25113 hyaB hybC hycA fdnG frdC ldhA backgrounds was not effective for enhancing hydrogen production and hydrogen yields (Tables 2 and 3). The reason may be that PoxB is more important under aerobic conditions (Abdel-Hamid et al. 2001). Note that E. coli cells require anaerobic conditions to synthesize hydrogen, as E. coli hydrogenases are sensitive to oxygen (Glick et al. 1980); therefore, PoxB product may not be important for enhanced hydrogen production. On the other hand, the inactivation of pyruvate dehydrogenase (AceE) was effective for enhancing both hydrogen production and hydrogen yield, although the effect is slight (BW25113 hyaB hybC hycA fdoG frdC ldhA vs BW25113 hyaB hybC hycA fdoG frdC ldhA aceE, Table 3); this may be caused by the increased metabolic flux to formate during glucose metabolism.
E. coli is robust because many technologies are available for its manipulation; for example, classical chemical mutagenesis followed by genome breeding (Patnaik et al. 2002), which may provide other important genes for enhanced hydrogen production, as there are indubitably unanticipated interactions in the metabolic pathways and their regulators. Microarray analysis (Maeda et al. 2007c) would then enable the molecular basis of the beneficial mutations to be discerned. Such approaches may hold promise for constructing even better strains for enhanced hydrogen production in glucose metabolism.
References
Abdel-Hamid AM, Attwood MM, Guest JR (2001) Pyruvate oxidase contributes to the aerobic growth efficiency of Escherichia coli. Microbiology 147:1483–1498
Andrews SC, Berks BC, McClay J, Ambler A, Quail MA, Golby P, Guest JR (1997) A 12-cistron Escherichia coli operon (hyf) encoding a putative proton-translocating formate hydrogenlyase system. Microbiology 143:3633–3647
Angelides KJ, Akiyama SK, Hammes GG (1979) Subunit stoichiometry and molecular weight of the pyruvate dehydrogenase multienzyme complex from Escherichia coli. Proc Natl Acad Sci U S A 76:3279–3283
Archana A, Sasikala C, Ramana Ch V (2003) Augmentation of H2 photoproduction in Rhodopseudomonas palustris by N-heterocyclic aromatic compounds. Biotechnol Lett 25:79–82
Axley MJ, Grahame DA, Stadtman TC (1990) Escherichia coli formate-hydrogen lyase. Purification and properties of the selenium-dependent formate dehydrogenase component. J Biol Chem 265:18213–18218
Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006–0008
Bagramyan K, Trchounian A (2003) Structural and functional features of formate hydrogen lyase, an enzyme of mixed-acid fermentation from Escherichia coli. Biochemistry (Mosc) 68: 1159–1170
Bertero MG, Rothery RA, Palak M, Hou C, Lim D, Blasco F, Weiner JH, Strynadka NC (2003) Insights into the respiratory electron transfer pathway from the structure of nitrate reductase A. Nat Struct Biol 10:681–687
Blattner FR, Plunkett G 3rd, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y (1997) The complete genome sequence of Escherichia coli K-12. Science 277:1453–1474
Böck A, Sawers G (1996) Cellular and Molecular Biology. In: Neidhardt FC, Curtiss JR II, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (eds) Escherichia coli and Salmonella. 2nd edn. ASM Press, Washington, pp 262–282
Cherepanov PP, Wackernagel W (1995) Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9–14
Das D, Veziroğlu TN (2001) Hydrogen production by biological processes: a survey of literature. Int J Hydrogen Energy 26:13–28
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97:6640–6645
Dunn S (2002) Hydrogen futures: toward a sustainable energy system. Int J Hydrogen Energy 27:235–264
Evans DJ, Pickett CJ (2003) Chemistry and the hydrogenases. Chem Soc Rev 32:268–275
Forzi L, Sawers RG (2007) Maturation of [NiFe]-hydrogenases in Escherichia coli. Biometals 20:565–578
Glick BR, Wang PY, Schneider H, Martin WG (1980) Identification and partial characterization of an Escherichia coli mutant with altered hydrogenase activity. Can J Biochem 58:361–367
Hansel A, Lindblad P (1998) Toward optimization of cyanobacteria as biotechnologically relevant producers of molecular hydrogen, a clean and renewable energy source. Appl Microbiol Biotechnol 50:153–160
Iverson TM, Luna-Chavez C, Cecchini G, Rees DC (1999) Structure of the Escherichia coli fumarate reductase respiratory complex. Science 284:1961–1966
Jung GY, Jung HO, Kim JR, Ahn Y, Park S (1999) Isolation and characterization of Rhodopseudomonas palustris P4 which utilizes CO with the production of H2. Biotechnol Lett 21:525–529
Kitagawa M, Ara T, Arifuzzaman M, Ioka-Nakamichi T, Inamoto E, Toyonaga H, Mori H (2005) Complete set of ORF clones of Escherichia coli ASKA library (A complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res 12:291–299
Klibanov AM, Alberti BN, Zale SE (1982) Enzymatic synthesis of formic acid from H2 and CO2 and production of hydrogen from formic acid. Biotechnol Bioeng 24:25–36
Kraemer JT, Bagley DM (2007) Improving the yield from fermentative hydrogen production. Biotechnol Lett 29:685–695
Lee SJ, Lee DY, Kim TY, Kim BH, Lee J, Lee SY (2005) Metabolic engineering of Escherichia coli for enhanced production of succinic acid, based on genome comparison and in silico gene knockout simulation. Appl Environ Microbiol 71:7880–7887
Maeda T, Sanchez-Torres V, Wood TK (2007a) Escherichia coli hydrogenase 3 is a reversible enzyme possessing hydrogen uptake and synthesis activities. Appl Microbiol Biotechnol 76:1035–1042
Maeda T, Sanchez-Torres V, Wood TK (2007b) Metabolic engineering to enhance bacterial hydrogen production. Microb Biotechnol. doi:https://doi.org/10.1111/j.1751-7915.2007.00003.x
Maeda T, Vardar G, Self WT, Wood TK (2007c) Inhibition of hydrogen uptake in Escherichia coli by expressing the hydrogenase from the cyanobacterium Synechocystis sp. PCC 6803. BMC Biotechnol 7:25
Mat-Jan F, Alam KY, Clark DP (1989) Mutants of Escherichia coli deficient in the fermentative lactate dehydrogenase. J Bacteriol 171:342–348
Minnan L, Jinli H, Xiaobin W, Huijuan X, Jinzao C, Chuannan L, Fengzhang Z, Liangshu X (2005) Isolation and characterization of a high H2-producing strain Klebsiella oxytoca HP1 from a hot spring. Res Microbiol 156:76–81
Oh Y-K, Seol E-H, Kim JR, Park S (2003) Fermentative biohydrogen by a new chemoheterotrophic bacterium Citrobacter sp. Y19. Int J Hydrogen Energy 28:1353–1359
Patnaik R, Louie S, Gavrilovic V, Perry K, Stemmer WP, Ryan CM, del Cardayré S (2002) Genome shuffling of Lactobacillus for improved acid tolerance. Nat Biotechnol 20:707–712
Penfold DW, Forster CF, Macaskie LE (2003) Increased hydrogen production by Escherichia coli strain HD701 in comparison with the wild-type parent strain MC4100. Enzyme Microb Technol 33:185–189
Penfold DW, Sargent F, Macaskie LE (2006) Inactivation of the Escherichia coli K-12 twin-arginine translocation system promotes increased hydrogen production. FEMS Microbiol Lett 262:135–137
Rachman MA, Furutani Y, Nakashimada Y, Kakizono T, Nishio N (1997) Enhanced hydrogen production in altered mixed acid fermentation of glucose by Enterobacter aerogenes. J Ferment Bioeng 83:358–363
Rossmann R, Sawers G, Böck A (1991) Mechanism of regulation of the formate-hydrogenlyase pathway by oxygen, nitrate, and pH: definition of the formate regulon. Mol Microbiol 5:2807–2814
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning, a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Schlensog V, Lutz S, Böck A (1994) Purification and DNA-binding properties of FHLA, the transcriptional activator of the formate hydrogenlyase system from Escherichia coli. J Biol Chem 269:19590–19596
Silhavy TJ, Berman ML, Enquist LW (1984) Experiments with gene fusions. Cold Spring Harbor Laboratories, Cold Spring Habor, NY
Sode K, Watanabe M, Makimoto H, Tomiyama M (1999) Construction and characterization of fermentative lactate dehydrogenase Escherichia coli mutant and its potential for bacterial hydrogen production. Appl Biochem Biotech 77–79:317–323
Suppmann B, Sawers G (1994) Isolation and characterization of hypophosphite-resistant mutants of Escherichia coli: identification of the FocA protein, encoded by the pfl operon, as a putative formate transporter. Mol Microbiol 11:965–982
Wang H, Gunsalus RP (2003) Coordinate regulation of the Escherichia coli formate dehydrogenase fdnGHI and fdhF genes in response to nitrate, nitrite, and formate: roles for NarL and NarP. J Bacteriol 185:5076–5085
Woods DD (1936) Hydrogenlyases: the synthesis of formic acid by bacteria. Biochem J 30:515–527
Yi KB, Harrison DP (2005) Low-pressure sorption-enhanced hydrogen production. Ind Eng Chem Res 44:1665–1669
Yoshida A, Nishimura T, Kawaguchi H, Inui M, Yukawa H (2005) Enhanced hydrogen production from formic acid by formate hydrogen lyase-overexpressing Escherichia coli strains. Appl Environ Microbiol 71:6762–6768
Yoshida A, Nishimura T, Kawaguchi H, Inui M, Yukawa H (2006) Enhanced hydrogen production from glucose using ldh- and frd-inactivated Escherichia coli strains. Appl Microbiol Biotechnol 73:67–72
Acknowledgment
The authors thank the National of Institute of Genetics, Japan, for sending the Keio and ASKA clones. This research was supported by DARPA (HR0011–06–1–0001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maeda, T., Sanchez-Torres, V. & Wood, T.K. Enhanced hydrogen production from glucose by metabolically engineered Escherichia coli . Appl Microbiol Biotechnol 77, 879–890 (2007). https://doi.org/10.1007/s00253-007-1217-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-1217-0