Abstract
Endophytic actinobacteria, which exist in the inner tissues of living plants, have attracted increasing attention among taxonomists, ecologists, agronomists, chemists and evolutionary biologists. Numerous studies have indicated that these prolific actinobacteria appear to have a capacity to produce an impressive array of secondary metabolites exhibiting a wide variety of biological activity, such as antibiotics, antitumor and anti-infection agents, plant growth promoters and enzymes, and may contribute to their host plants by promoting growth and enhancing their ability of withstanding the environmental stresses. These microorganisms may represent an underexplored reservoir of novel species of potential interest in the discovery of novel lead compounds and for exploitation in pharmaceutical, agriculture and industry. This review focuses on new findings in the isolation methods, bio- and chemical diversity of endophytic actinobacteria and reveals the potential biotechnological application. The facing problems and strategies for biodiversity research and bioactive natural products producing are also discussed.
Similar content being viewed by others
Introduction
Over the last several decades, natural products have continued to play a highly significant role in the drug discovery and development process. As reviewed by Newman and Cragg (2007), about 28% of the new chemical entities and 42% of the anticancer drugs introduced into the market worldwide from 1981 to 2006 were natural products and their derivatives. Besides plants, microorganisms represent a rich source of bioactive metabolites. More than 22,000 biologically active compounds have been obtained from microbes by the end of 2002. Among them, 45% were produced by actinobacteria, especially the excellent producers in the genus Streptomyces (Bérdy 2005). Actinobacteria have made a phenomenal contribution to the health and well-being of people throughout the world (Demain and Sanchez 2009). The emergence of antibiotics resistance developed in bacterial pathogens and the current increase in the number of new diseases and pathogens, such as acquired immunodeficiency syndrome, severe acute respiratory syndrome and H1N1 flu virus has caused a resurgence of interest in finding new biologically active compounds for drug discovery. However, for many years intensive screening of soil born microbes, the frequency of discovering structurally new compounds is apparently decreasing (Bérdy 2005). Thus, unexplored and new microbial habitats need to be examined for microbial resources that produce useful bioactive compounds.
One relatively overlooked and promising niche is the inner tissues of higher plants. Early studies have demonstrated that some actinobacteria can form intimate associations with plants and colonize their inner tissues. Frankia species and Streptomyces scabies can penetrate their host and establish either endophytic or pathogenic associations (Benson and Silvester 1993; Doumbou et al. 1998). The actinomycete bacteria that reside in the tissue of living plants and do not visibly harm the plants are known as endophytic actinobacteria (Stone et al. 2000). These microbes live in different organs (roots, stems, leaves, flowers, fruits and seeds) of the host plants, mainly in inter or intracellular spaces. It is noteworthy that, of the nearly 300,000 plant species on the earth, each individual plant is considered to host one or more type of endophytes (Strobel and Daisy 2003), creating an enormous biodiversity. However, only a few of these plants-associated endophytic actinobacteria have been studied, indicating the opportunity to find interesting species and related natural products among myriads of plants in different niches and ecosystems is great. Recent studies have revealed a large richness of endophytic actinobacterial species and diverse compounds with different functions (Araujo et al. 2002; Coombs and Franco 2003; Ryan et al. 2008; Bascom-Slack et al. 2009). In some cases, they can act as biological control agents (Cao et al. 2005), enhance plant growth (Igarashi et al. 2002) and promote plant establishment under adverse conditions (Hasegawa et al. 2006). These actinobacteria are relatively unstudied and potential sources of novel natural products for exploitation in medicine, agriculture, and industry (Strobel et al. 2004). The goals of this review are to summarize isolation and cultivation methods and new findings in the recent study of the astounding endophytic actinobacterial diversity and discuss the enormous biotechnological potential in the areas of natural products discovery and related applications.
Isolation and cultivation methods for endophytic actinobacteria
Early in the year 1886, the genus Frankia was isolated from non-legume root nodules, indicating that actinomycetes can closely be associated with plants (Okazaki 2003). For the last two decades, non-Frankia endophytic actinobacteria have been isolated from almost all vascular plants examined, ranging from woody species to herbaceous plants. It is evident that colonization of terrestrial plants by actinobacteria is ubiquitous and common in nature. Thus, endophytic actinobacteria are important components of microbial biodiversity because of the numerous plant species. Although, theoretically, each plant can be selected for actinobacteria isolation. Strobel and Daisy (2003) stressed that plant selection is tactical. Those plants with an unconventional setting and biology as well as with established ethnobotanic values would be preferred and promising sources of endophytes producing novel bioactive products. A specific protocol for isolation of actinobacteria from a given plant is important, as isolation represents the most crucial step during the process of obtaining pure cultures, and the fact that host species, sampling strategy, host-endophyte and inter-endophyte interations, tissue type and ages, geographic and habitat distribution, culture conditions, surface sterilants and selective media all influence the detection and enumeration of endophytes (Zhang et al. 2006). Some detailed isolation methods and procedures, including plant sampling, surface sterilization and used media have been reviewed (Hallmann et al. 2006) and introduced by Coombs and Franco (2003).
Surface sterilization is the first and obligatory step for endophyte isolation in order to kill all the surface microbes. It is usually accomplished by treatment of plant tissues with oxidant or general sterilant for a period, followed by a sterile rinse. Commonly used surface disinfectants are ethanol (70–95%), sodium hypochlorite (3–10%) and hydrogen peroxide. Some surfactants such as Tween 20, Tween 80 and Triton X-100 can be added to enhance surface sterilization effectiveness (Sturz 1995; Hallmann et al. 2006). A common protocol involves a three-step procedure as described by Coombs and Franco (2003). We recommend a five-step procedure, and adding sodium thiosulfate solution after being treated by sodium hypochlorite to improve cultivation efficiency on media plates, due to the fact that thiosulfate can suppress the detrimental effects of residual NaOCl on plant material surfaces, which may kill endophytes or at least stressed them so much that they become unable to form colonies on the plates (Qin et al. 2009b). After the treatment, plant tissues can be soaked in 10% NaHCO3 solution in order to inhibit the endophytic fungi, which could overgrow the samples and mask the actinobacteria (Nimnoi et al. 2010). After each treatment, validation of surface sterilization should be checked to prove that the epiphytic microorganisms have been killed and that subsequent isolates are true endophytes. In general, the sterilization procedure should be optimized for each plant tissues, especially the sterilization time, since the sensitivity varies with plant species, age and organs.
Pretreatment of plant issues is an important step for isolation of endophytic actinobacteria. Surface-sterilized plant samples often need drying at 80°C or 100°C for 15 to 30 min to kill bacteria. Commonly, materials were aseptically sectioned into small fragments about 0.2 × 1.0 cm (Coombs and Franco 2003; Cao et al. 2004; Verma et al. 2009a, b; Fialho de Oliveira et al. 2010), then distributed onto isolation media. On this basis, tissues can be aseptically crumbled into smaller fragments by commercial blender (Qin et al. 2008a, b; Li et al. 2009d), which enlarged the colonization area of samples onto the media agar, thus making it helpful for recovering endophytes. Tender and soft tissues can be pestled and homogenized in a mortar with extraction solution or buffer and then use gradient dilution method. Maceration, vacuum and pressure bomb techniques have been employed for isolating gram-positive species (Hallmann et al. 1997a, b). These two preferred methods could recover a higher number of less commonly detected genera. We also recommend the newly applied different combined enzymatic hydrolysis and differential centrifugation bacterial cell enrichment methods (Jiao et al. 2006; Qin et al. 2009b; Ikeda et al. 2009). These techniques collected most of the microbes associated with plant tissues and maintained the microbial cells intact, and were especially useful for isolating rare endophytic actinobacteria. In a word, to make endophytes complete release from inner parts of plant material is a significant step for pure culture isolation. Based on this rationale, some other innovative pre-treatment methods can be explored.
Growth of microbes in the laboratory is dependent on the composition of the media and the cultivation conditions that are applied. For endophytic actinobacteria, some classical media for soil actinomycetes isolation, such as humic acid-vitamin (HV) (Hayakawa 1990), International Streptomyces Project media ISP 2 and ISP 5 (Shirling and Gottlieb 1966), raffinose-histidine agar (Vickers et al. 1984) and starch casein agar (Küster and Williams 1964) are also available. Low nutrient medium TWYE was found effective for isolation of endophytic actinobacteria (Coombs and Franco 2003; Qin et al. 2009b; Li et al. 2009a), due to the fact that high nutrient concentration allowed fast growing bacteria to overgrow slower growing actinobacteria. Recently, by our large-scale isolation investigations of tropical rainforest plants, some media composed of amino acids (proline, arginine, and asparagine) as nitrogen sources and cellulose, xylan, sodium propionate, sodium succinate as carbon sources had prominent isolation effectiveness, especially for some uncommon rare endophytic actinobacterial genera (Qin et al. 2009b). This could be explained by the fact that in many plants amino acids account for the major nitrogen, and cellulose, xylan are the components of plant cell wall. Sometimes, different pre-treatment methods and media combinations had different isolation effectiveness. We also found that adding a certain amount of plant extracts into the isolation medium is effective. This may be due to the different physiological properties of some actinomycetes in plant tissues and soils (Okazaki 2003). At present, understanding the physiological properties of endophytic actinobacteria seems difficult because of the complicated symbiotic relationships. By simulating the micro-environments of inner plants to design media is a good strategy for isolation.
Biodiversity of endophytic actinobacteria
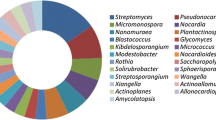
Endophytic actinobacteria have been isolated from a variety of healthy plant species ranging from crop plants, such as wheat, rice, potato, carrots, tomato and citrus (Nejad and Johnson 2000; Araujo et al. 2002; Coombs and Franco 2003; Surette et al. 2003; Sessitsch et al. 2004; Tian et al. 2007; Velazquez et al. 2008), different woody tree species (Taechowisan et al. 2003; Zin et al. 2007; Yuan et al. 2008; Zhao et al. 2010a, b, c), ferns and club mosses (Janso and Carter 2010). In general, Streptomyces spp. were the most predominant species and Microbispora, Micromonospora, Nocardioides, Nocardia and Streptosporangium are the common genera. For instance, 619 actinomycetes were isolated from different cultivars of tomato, and all of them were Streptomyces spp. (Tan et al. 2006). From 36 medicinal plant species of Thailand, Taechowisan et al. (2003) isolated 330 strains belonging to four different genera (Streptomyces, Microbispora, Nocardia and Micromonospora). Lee et al. (2008) isolated 81 endophytic actinobacteria including eight genera from Chinese cabbage roots, and Microbispora spp. were the most common isolates, followed by Streptomyces spp. and Micromonospora spp. Relative to stems and leaves, roots harboured more diverse actinobacterial populations. To date, more than 40 new taxa have been found by polyphasic taxonomic approaches, including four new genera, Plantactinospora, Actinophytocola, Phytohabitans and Jishengella (Table 1). Most of these published species had more than 97% similarities with recognized nearest relatives based on the analysis of the 16S rRNA gene sequences with closest non-endophytic neighbours. In addition, few species possessed unique rRNA gene sequences, which probably had experienced long-time evolution. This may presume that some actinobacterial endophytes are very similar with non-endophytic isolates, especially those from soils. In fact, the endophyte population was considered to be a subset of the rhizosphere popolation and soil type, soil microbial population significantly affects the endophytic actinobacterial communities (Conn and Franco 2004; Compant et al. 2010).
Strobel and Daisy (2003) believed that the greatest diversity of endophytes is likely to occur in the tropical and temperature regions. Janso and Carter (2010) isolated 123 endophytic actinomycetes from tropical plants collected from several locations in Papua New Guinea and Mborokua Island, Solomon Islands. 16S rDNA sequence analysis revealed that 17 different genera were represented and rare genera such as Sphaerisporangium and Planotetraspora, which have never been previously reported to be endophytic, were quite prevalent. Phylogenetic analyses suggested that the many endophytic strains in Thermomonosporaceae and Micromonosporaceae may represent new genera. Tropical rainforests possessing the greatest biodiversity on the earth have the prospect of housing endophytes with great biodiversity. In our study, 2,174 endophytic actinobacteria were isolated from different medicinal plants in Xishuangbanna tropical rainforest of China by using diverse pretreatment methods and selective media, and they represent an unexpected variety of ten different suborders and 32 genera, including at least 19 new taxa (Qin et al. 2009b). Only from one kind of plant, Maytenus austroyunnanensis, a new genus and seven new species were isolated. Obviously, the actinobacterial richness and diversity from tropical rainforest are much higher comparison with other regions, and tropical rainforest have potential as promising source of actinobacteria and new species. The endophytic actinobacterial communities are diverse and the extent of diversity may vary between different sample collection regions and different plant species. The diversity of genera and the number of culturable endophytic actinobacteria was largely dependent on the isolation methods.
It has been estimated that less than 1% of bacteria species are currently known (Davis et al. 2005), indicating that millions of microbial species remain to be discovered. The applications of 16S rRNA gene-based culture-independent molecular approaches, such as polymerase chain reaction (PCR)-based 16S rRNA gene clone library, denaturing gradient gel electrophoresis and terminal restriction fragment length polymorphism (T-RFLP) analysis are useful to reveal the complex microbial community inhabiting various plants. Sometimes, the combination of culturing methods and culture-independent analysis is needed for the study of endophytic community. Conn and Franco (2004) investigated the wheat root actinobacteria using the T-RFLP method and revealed a large diversity than that obtained by culturing. The presence of endophytic actinobacteria from rice was studied by Tian et al. (2007), and their results suggest diverse communities within stems and roots. Jiao et al. (2006) firstly introduced the idea of enriching uncultured bacterial cells from plant tissues by enzymatic hydrolysis of the plant cell wall, followed by differential centrifugation. Subsequently, Wang et al. (2008) reported the diversity of uncultured microbes associated with medicinal plant Mallotus nudiflorus using a modified method, and found that actinobacteria were the most dominant microbial group, covering 37.7% in the 16S rRNA gene library. This enzymatic hydrolysis and differential centrifugation microbial enrichment method has also been carried out to study the endophytic bacterial diversity in grapevine leaf tissues (Bulgari et al. 2009). Another technique suitable for enriching bacterial cells from fresh plant tissues was developed (Ikeda et al. 2009) and has been successfully applied to analyze the clone libraries of 16S rRNA gene and clarify the diversity of endophytic actinobacterial communities in stems and leaves of soybeans and rice (Ikeda et al. 2009, 2010). We studied the composition of endophytic actinobacteria associated with M. austroyunnanensis using both culture-based and culture-independent techniques. In total, 20 different genera were isolated from roots, stems and leaves, with Streptomyces spp. the most predominant species, whereas more than 30 genera and uncultured actinobacteria were detected from the 16S rRNA gene clone library using the cell enrichment method, and the culturable community composition was different from that of the 16S rRNA gene clone library (unpublished data). However, detection limits of these techniques still exist and may prevent the identification of many heterogeneous species. Recently developed high-throughput sequencing technologies, including those commercialized by 454 Life Sciences/Roche Applied Sciences (454), Illumina Incorporated (Solexa), Applied Biosciences (SOLiD), Dover Systems (Polonator), and Helicos Biosciences Corporation have allowed for rapid sequencing of whole genomes and been used to explore a variety of microbial ecological communities (Mardis 2008; Shendure and Ji 2008; Miller et al. 2009; Lauber et al. 2010; Robinson et al. 2010). In 2010, pyrosequencing has also been used for the first time to examine the bacterial endophyte community in the roots of 12 different potato cultivars revealing an unprecedented level of bacterial root endophytes (Manter et al. 2010). These discoveries greatly improved our understanding of the ecological distributions and the complexity of plant-associated actinobacteria. However, the actual number and diversity of endophytic actinobacteria are enormous and a majority remains unknown.
Bioactive natural products and biotechnological potential
From a drug discovery point of view, the novel actinobacterial strains are attractive, as they are likely to contain new genes in theory and held promising for novel products, thus the chance of finding novel pharmaceutical bioactive compounds from endophytic actinobacteria is considerable. Endophytic actinobacteria associated with traditionally used medicinal plants especially of the tropics could be a rich source of functional metabolites (Strobel et al. 2004). Current interest in natural products from endophytes especially endophytic fungi is evident from the number of review articles that have appeared in the recent literatures (Hasegawa et al. 2006; Zhang et al. 2006; Gunatilaka 2006; Guo et al. 2008; Staniek et al. 2008; Ryan et al. 2008; Verma et al. 2009a, b). Recent studies of the culturable endophytic actinobacteria have resulted in the identification of many new natural products with diverse biological activities (Table 2). This review focuses particularly on the recent advances up to the writing time of 2010. In this section, we mainly review the bioactive compounds from endophytic actinobacteria and their biotechnological potential applications. Some examples are listed below.
Antibiotics from endophytic actinobacteria
Many endophytic actinobacteria, especially those from medicinal plants possess the ability of inhibiting or killing a wide variety of harmful microorganisms like pathogenic bacteria, fungi and viruses. Thus, there is great application value to develop antimicrobial drugs from endophytic actinobacteria. Hitherto, a lot of new antibiotics have been isolated, such as munumbicins A-D (Castillo et al. 2002), celastramycins A-B (Pullen et al. 2002), kakadumycins (Castillo et al. 2003) and demethylnovobiocins (Igarashi 2004). From the culture broth of endophytic strain Streptomyces sp. TP-A0595, a simple compound 6-prenylindole was isolated and exhibited significant antifungal activity against plant pathogen Fusarium oxysporum. 6-Prenylindole was originally isolated from the plant liverwort (Hepaticae), and this is an additional example of the isolation of the same compound from plant and endophyte (Igarashi 2004). Two novel compounds cedarmycins A and B were isolated from the strain Streptomyces sp. TP-A0456, which was isolated from a twig of cedar. Cedarmycins A showed in vitro antifungal activity against Candida glabrata with the MIC value of 0.4 μg/ml (Igarashi 2004). An endophytic Streptomyces sp. Tc022 isolated from roots of Alpinia galanga strongly inhibited Colletotrichum musae and Candida albicans. Extraction of the culture medium of Streptomyces sp. Tc022 afforded a major component actinomycin D, which displayed very strong antifungal activity (Taechowisan et al. 2006). Castillo et al. (2006) isolated two new chromophoric peptides antibiotics, designated munumbicins E-4 and E-5 from endophytic Streptomyces NRRL 30562, which also produced broad-spectrum antibiotics munumbicins A-D. Both compounds showed broad-spectrum activity against gram-positive and gram-negative bacteria. Recently, a new antimycotic compound saadamycin was isolated from endophytic Streptomyces sp. Hedaya48, and it exhibited significant antimycotic activity against dermatophytes and other clinical fungi (El-Gendy and EL-Bondkly 2010). These studies listed above, reinforced the assumption that endophytic actinobacteria could be a promising source of antimicrobial substances.
Antitumor and anti-inflammatory agents from endophytic actinobacteria
In recent years, there has been an increasing interest in searching antitumor agents from endophytes. Considering that endophytic actinobacteria live in close association with their host plants and the long co-evolution relationship, there is a real possibility that genes involved in natural products biosynthesis could be exchanged via horizontal gene transfer (HGT) between microbes and plants, resulting in production of plant-derived compounds by a microbe such as the paclitaxel-producing Kitasatospora sp. isolated from Taxus baccata in Italy (Caruso et al. 2000; Janso and Carter 2010). This is the first report of production of taxol from endophytic actinomycetes. Another example is the maytansinoids (19-membered macrocyclic lactams related to ansamycin antibiotics), extraordinarily potent antitumor agents that were originally isolated from members of the higher plant families Celastraceae, Rhamnaceae and Euphorbiaceae (Kupchan et al. 1972; Powel et al. 1982), as well as some mosses (Suwanborirux et al. 1990), and, remarkably, from plant-associated actinomycete Actinosynnema pretiosum (Higashide et al. 1977). Noticeably, one novel chlorine-containing ansamycin, namely naphthomycin K (Fig. 1a), was isolated from the endophytic strain Streptomyces sp. CS of the maytansinoids producer medicinal plant Maytenus hookeri. It showed evident cytotoxic activity against P388 and A-549 cell lines at IC50 0.07 and 3.17 μM, but no inhibitory activities against Staphylococcus aureus and Mycobacterium tuberculosis (Lu and Shen 2007). Interestingly, ansacarbamitocins, a new family of maytansinoids, were reported from a soil actinomycete strain Amycolatopsis CP2808 which belongs to the family Pseudonocardiaceae, the same family as the ansamitocin-producing strain A. pretiosum (Snipes et al. 2007). Strain Streptomyces sp. CS was an outstanding bioactive compounds producer. Previously, 24-demethyl-bafilomycin C1, a new member of the bafilomycin subfamily and two more new bafilomycin derivatives were isolated from the this strain (Lu and Shen 2003, 2004). During the subsequent research, five new 16-membered macrolides, belonging to the bafilomycin subfamily were isolated and showed cytotoxic activity against MDA-MB-435 cell line in vitro (Li et al. 2010a). From the strain Streptomyces sp. ls9131, which was also isolated from M. hookeri, two novel macrolides were found. Bioassay results showed that the compound dimeric dinactin (Fig. 1b) had strong antineoplastic activity and antibacterial activity (Zhao et al. 2005). Two novel anthraquinones, lupinacidins A and B (Fig. 1c), were isolated from the fermentation broth of a new endophytic actinomycete Micromonospora lupine. Lupinacidins were found to show significant inhibitory effects on the invasion of murine colon 26-L5 carcinoma cells (Igarashi et al. 2007). Kim et al. (2006) isolated two new 6-alkylsalicylic acids, salaceyins A and B from another strain Streptomyces laceyi MS53 and their structures were determined on the basis of spectroscopic data (Fig. 1d). Salaceyins A and B exhibited cytotoxicity against a human breast cancer cell line (SKBR3) with IC50 values of 3.0 and 5.5 μg/ml, respectively. A new cytotoxic compound, pterocidin (Fig. 1e), isolated from the endophytic Streptomyces hygroscopicus TP-A0451, showed cytotoxicity against some human cancer cell lines with IC50 values of 2.9–7.1 μM (Igarashi et al. 2006).
Recently, two compounds 5, 7-dimethoxy-4-phenylcoumarin (Fig. 1f) and 5, 7-dimethoxy-4-p-methoxylphenylcoumarin, originally produced by numerous species of plants were isolated from endophytic Streptomyces aureofaciens CMUAc130 and showed to have antifungal and antitumor activity (Taechowisan et al. 2005, 2007a). They were also investigated for their effects not only on the formation of nitric oxide (NO), prostaglandin E2 (PGE2) and tumour necrosis factor (TNF-α), but also on inducible nitric oxide synthase and cyclooxygenase-2 in lipopolysaccharide (LPS)-induced murine macrophage RAW 264.7 cells. The inhibitory effects were shown in concentration-dependent manners. They significantly reduced the formation of TNF-α. These findings support the application of them as anti-inflammatory agent (Taechowisan et al. 2007b). From the endophytic strain Streptomyces sp. SUC1, four novel secondary metabolites, lansai A–D were isolated. Lansai B showed weakly active against the BC cell line (IC50 15.03 μg/ml) (Tuntiwachwuttikul et al. 2008), lansai C also showed significant anti-inflammatory activity in LPS-induced RAW 264.7 cells (Taechowisana et al. 2009). In a word, endophytic actinobacteria still remain a relatively untapped source of novel natural products, presumed to push forward the frontiers of drug discovery.
Other pharmaceutical compounds from endophytic actinobacteria
Endophytic actinobacteria also produced other bioactive compounds with diverse functions different from those discussed above. An endophytic Streptomyces sp. (AC-2), isolated from a traditional Chinese medicine plant Cistanche deserticola Y. C. Ma, produced tyrosol. This compound can promote an increase of intracellular cAMP special on GPR12 transfected cells, such as CHO and HEK293, might be a new possible ligand for GPR12 (Lin et al. 2008). A novel peptide compound coronamycin was purified from a verticillate Streptomyces sp. (MSU-2110) colonizing an epiphytic vine, Monstera sp. It exhibited remarkable active against the malarial parasite, Plasmodium falciparum, with an IC50 of 9.0 ng/ml. In addition, it showed strong activity against pythiaceous fungi and the human fungal pathogen Cryptococcus neoformans (Ezra et al. 2004). These natural bioactive metabolites with multifold applications expand actinobacterial use arenas. Analysing the endophytic actinobacterial born compounds and their hosts (Table 2), a correlation seems to exist between their ecological basis and bioactive metabolites. Endophytic actinomycetes in higher plants, particularly those associated with medicinal plants and being used traditional medicines, are more likely to contain bioactive compounds. This principle is supported by the examples of taxol and maytansinoids as discussed above in this review. Therefore, to search for novel compounds could be directed towards plants that commonly serve indigenous populations for medicinal purposes and plants growing in unique environmental setting or interesting endemic locations as they are expected to harbour novel endophytes that may produce unique metabolites with diversified applications (Strobel and Daisy 2003).
Biological control agents
In the recent years, endophytic actinobacteria have attracted the attention of researchers as biological control agents of plant pathogens due to their plant colonizing ability and antifungal activities. They have been shown to protect plants against different soil-borne plant pathogens, including Rhizoctonia solani, Verticillium dahliae, Plectosporium tabacinum, Gaeumannomyces graminis var. tritici, F. oxysporum, Pythium aphanidermatum and Colletotrichum orbiculare (Krechel et al. 2002; El-Tarabily 2003; Coombs et al. 2004; Cao et al. 2005; El-Tarabily et al. 2009; Shimizu et al. 2009). Endophytic actinobacteria and their role as biocontrol agents have been partly discussed (Hasegawa et al. 2006).
Studies on the mechanisms of action of these endophytes have focused mainly on the production of bioactive compounds, such as antibiotics, cell wall degrading enzyme and competition for nutrients (El-Tarabily and Sivasithamparam 2006). In addition, endophytic actinobacteria have also been found to hold the ability of triggering plant induced systemic resistance (ISR). The ‘versatile’ endophytic strain Streptomyces galbus R-5 not only released cellulose, pectinase, produced actinomycin X 2 and fungichromin to induce resistance in the rhododendron seedlings, but also triggered plant jasmonate-associated defence responses (Shimizu et al. 2005). Conn et al. (2008) observed that seed inoculations with endophytic Streptomyces sp. EN27 and Micromonospora sp. strain EN43 led to increased resistance in Arabidopsis thaliana leaves against pathogens, Erwinia carotovora and F. oxysporum, and triggered the expression of defence genes related to SA- or jasmonic acid/ethylene-dependent signalling pathways in the absence of a pathogen. Moreover, culture filtrates obtained from cells of Micromonospora sp. strain EN43 grown in either minimal or rich medium activated the system acquired resistance and jasmonates/ethylene pathways, respectively, indicating that two different sets of metabolites are synthesized by this endophytic actinobacteria for eliciting the plant defense pathway (Hirsch and Valdés 2010). At present, whether or not these metabolites are antibiotics or other molecules is not known. The intimate association of endophytic actinobacteria with plants and born bioactive natural products offers a unique opportunity to find effective drugs or biofungicides for potential application in plant protection and biological control.
Plant growth promoting agents
Nowadays, increased public concern about environmental problems caused either directly or indirectly by the use of fertilizers, pesticides, herbicides, and fungicides, has prompted researchers to consider alternatives to these established chemical strategies for facilitating plant growth in agriculture, horticulture, and silviculture (Glick et al. 2007). Endophytic actinobacteria are of special interest since they possess many properties that could benefit to plant growth. The plant growth promoting actinobacterial endophytes possess similar mechanisms for one of the following functions: production biological control agents, or production of plant growth promotion compounds, such as auxins, cytokinins and gibberellins, or producing siderophore to bind Fe3+ from the environment and help to improve nutrient uptake, supply of plant nutrients (nitrogen, phosphate and other mineral nutrients), or suppression of stress ethylene production by 1-aminocyclopropane-1-carboxylate (ACC) deaminase activity (Compant et al. 2005; Kannan and Sureendar 2008; Sun et al. 2009; Nimnoi et al. 2010). For example, several endophytic actinobacteria isolated from winter rye produced indolyl-3-acetic acid. Treatment of winter rye seeds with auxin producing strains increased the germination capacity and enhanced an intensive seedling growth in vitro (Merzaeva and Shirokikh 2010). Two compounds pteridic acids A and B were found from species S. hygroscopicus TP-A0451 isolated from a stem of bracken, Pteridium aquilinum (Igarashi 2004). Pteridic acids inhibited the rice germination at 100 ppm, but pteridic acid A promoted the root elongation at 20 ppm. Futhermore, pteridic acid A induced the adventitious root formation of the kidney bean hypocotyls at 1 nM as effectively as indoleacetic acid. Plant growth-promoting properties of endophytic actinobacteria and the recent increased understanding of some of the mechanisms suggest that this promising source merits further investigations for potential application in agriculture.
Facing problems and strategies
The recent advances discussed above have dramatically enlarged our knowledge on the diversity and biotechnological potential of plant-associated endophytic actinobacteria. However, many questions regarding to the ecological function of actinobacteria in the endophytic environments as well as their evolution and biogeographic distribution remain to be answered. Sustainable exploration of biotechnological potentials of these microorganisms has been limited due to the re-isolation of known strains and products, and many problems still exist in the following areas, needed to be resolved.
Firstly, what is the ‘real’ endophytic actinobacteria? Did endophytic actinobacteria evolve from free-living counterparts (terrestrial soil or even marine micoorganisms) through gene loss and acquisition? What are the specific genotypes that make up the endophytic obligate phenotype, or are there specific endophytic actinobacterial genetic markers that will aid in the definition of endophytic actinobacteria? Do endophytic actinobacteria produce plant derived and other compounds that are not found in terrestrial relatives, or what’s difference between them? The endophyte niche is a hot spot for horizontal gene transfer. At present, the HGT hypothesis gives us a plausible alternative postulating the endophyte-host co-evolution. The release of genomes from endophtes will enable comparative genomics studies to better address questions concerning the evolution and ecology basis of endophytic actinobacteria. For instance, Krause et al. (2006) reported the first full genome sequence of an endophyte, strain BH72 of Azoarcus species, and this sequence provided valuable insights into the life of bacterial endophytes, including information about interactions with host plants. From the new study of Wu et al. (2010), comparative genomics provided a powerful tool to gain new insights into the niche adaptation of different species of Pseudomonas putida (from endophytic and soil) to specific lifestyles and environmental niches, and clearly demonstrated that horizontal gene transfer played a key role in this adaptation process, as many of the niche-specific functions were found to be encoded on clearly defined genomic islands. Clarification and answer of the above-listed issues will demask the real endophytic actinobacteria and guide future rational search and discovery programmes.
Secondly, although collection plants is relatively inexpensive and easy, little is known about the endophytic actinobacterial distribution and abundance compared to soil actinomycetes, and the vast majority of endophytic actinobacteria remain unexplored and unknown. How to isolate more endophytic actinobacterial strains, especially rare actinobacteria and new species? To solve this problem, new isolation strategies and media must be introduced. Four cultivation principles and methods merit particular attention: making endophytes complete release from inner tissues of plant samples and cells enrichment; designing media through simulating host plant nature environments; low nutrient concentration; and extended incubation times. Among the vast plant kindom, only a handful of plants have been studied. In addition to the tropical rain forests, those plants growing in harsh habitats such as hot and cold deserts, saline and acidic soils and marine habitats, including algae, bryophytes and lichens should be isolated and screened for bioactive metabolite producing endophytic actinobacteria (Suryanarayanan et al. 2009). New isolation procedures and special habitats plants open a possibility of isolation of new and potential products and processes from them. An issue of concern, which has not been addressed in this review is the Endophytic actinobacterial biogeography. Do endophytic actinobacterial biogeographical distribution and spatial variation reflects the proposition that “the environmental selects”? Understanding the biogeography is not simple of academic interest but also provides a actinobacterial map for biodiscovery.
Third, vast number of uncultured endophytic actinobacteria should not be overlooked. How to cultivate and utilize these microorganisms? Can we find new compounds and genes from uncultured endophytic actinobacteria? The combination of both cultivation-dependent and cultivation-independent techniques in the same study is recommended because cultivation-based techniques enable the recovery and testing of isolates, whereas cultivation-independent techniques enable the screening for variations in the total endophytic communities (Van Overbeek and Van Elsas 2008). The information obtained from culture-independent studies will not only disclose the presence and distribution of endophytic actinobacteria, but also could be used to design selective isolation schemes to cultivate more endophytic actinobacteria, including novel taxa from the same samples. Culturing the previously uncultured endophytic actinobacteria should use some new approaches, such as placing cells in chambers that allow diffusion of compounds from the natural environment, traps enclosed with porous membranes that specifically capture hyphae-forming actimycetes and growth in the presence of cultivable helper species (Lewis et al. 2010), and this would represent a unique and promising source for the discovery of novel secondary metabolites. Metagenomics and combined high-throughput screening of total DNA from environmental samples also provides an alternative way of discovering new antibiotics and biosynthetic genes. The first metagenomic fosmid library of endophytes was successfully constructed by Wang et al. (2008), which had 1.37 × 106 clones. For example, new gene clusters such as polyketides synthases (PKS) and nonribosomal peptide synthases (NRPS) found in the library indicate the likelihood of novel compounds being produced. This work paved the way for recovery and biochemical characterization of endophytic actinobacterial functional gene repertoire.
Finally, how to quickly find new bioactive secondary metabolites from numerous endophytic actinobacteria? Actinobacterial taxonomic diversity can be used as a surrogate for chemical diversity. As reviewed by Goodfellow and Fiedler (2010), application of a bioprospecting strategy that a combination of selective isolation, strain dereplication and screening procedures can lead to the discovery of new natural products from novel actinomycetes isolated from geographically diverse samples. As complete sequences of many biosynthetic gene clusters related to different kinds of antibiotics produced by actinobacteria have been gained, it is possible to quickly screen strains that have genes involved in the synthesis of secondary metabolites (Ayuso-Sacido and Genilloud 2004). Additionally, the phylogenetic analysis of the amplified synthase genes can help us to make prediction about the strains that could biosynthesize new compounds with related structural characters. This would open up the possibility of using high-throughput PCR screening method to detect the antibiotic produced endophytic actinobacterial strains. For example, by combined bioactive assay screening, two endophytic Streptomyces strains isolated from maytansinoids producer Trewia nudiflora Linn were proved to have the potential of producing ansamycins, which further enhanced the hypothesis that endophytes might be involved in the biosynthesis of plant maytansinoids (Zhu et al. 2009). In fact, many other genetic manipulation techniques applied for soil or marine actinomycetes are also available. For example, reengineering artifical or hybrid PKSS biosynthetic pathways to produce new chemical entities is a reality. Natural gene shuffling, combinatorial biosynthesis, transcriptome analysis and heterologous expression, coupled with recent achievements of genomic sciences, would accelerate the novel natural products discovery from endophytic actinobacteria (Moore et al. 2005; Baltz 2008).
Concluding remarks and perspectives
The past decade have seen a dramatically increase of information in the field of novel species and bioactive compounds isolated from plant associated endophytes. It is therefore necessary to timely review the past successes of endophytic actinobacterial born natural products discovery and to examine future prospects to further explore the biodiversity of endophytic actinobacteria and their produced bioactive secondary metabolites for biotechnological applications.
As reviewed above, the endophytic environment, without any doubt, is keeping a myriad of new actinobacteria providing novel structural diversity to be discovered and used in pharmaceutical, agriculture, and industry. Even so, the study on endophytic actinobacteria is just beginning. We are in the early stages of a renaissance in natural product discovery from endophytic actinobacteria. Future success not only relies on continued investigations of endophytic actinobacterial resources from wide habitats, but also dependents on applied new technologies. Recent applied metagenomic approaches, genome scanning and whole genome sequence mining (Hornung et al. 2007) provide efficient ways for screening gene cluster involved in the biosynthesis of pharmaceutical metabolites and revelation of biosynthesis mechanisms from undiscovered endophytic actinobacteria. Advances in metabolic engineering, combinatorial biosynthesis and heterologous expression will lead to the discovery of new pharmaceutical compounds from endophytic actinobacteria, hopefully with improved therapeutic properties. More successes of whole-genome sequencing and proteomic studies of key endophytes will enhance our understanding of the complicated plant-endophyte interactions and mechanisms and enable better biotechnological applications in plant biocontrol, growth promoting and other areas. We also suggest comprehensive cooperation among global taxonomists, ecologists, natural product chemists, agronomists and bioengineers to better exploit their biodiversity and biotechnological potential.
References
Araujo WL, Marcon J, WJr M, Van Elsas JD, JWVan V, Azevedo JL (2002) Diversity of endophytic bacterial populations and their interaction with Xylella fastidiosa in citrus plants. Appl Environ Microbiol 68:4906–4914
Ayuso-Sacido A, Genilloud O (2004) New PCR primers for the screening of NRPS and PKS-I system in actinomycetes: detection and distribution of these biosynthetic gene sequences in major taxonomic groups. Microb Ecol 49:10–24
Baltz RH (2008) Renaissance in antibacterial discovery from actinomycetes. Curr Opin Pharmacol 8:557–563
Bascom-Slack CA, Ma C, Moore E, Babbs B, Fenn K, Greene JS, Hann BD, Keehner J, Kelley-Swift EG, Kembaiyan V, Lee SJ, Li P, Light DY, Lin EH, Schorn MA, Vekhter D, Boulanger LA, Hess WM, Vargas PN, Strobel GA, Strobel SA (2009) Multiple, novel biologically active endophytic actinomycetes isolated from upper Amazonian rainforests. Microb Ecol 58(2):374–383
Benson DR, Silvester WB (1993) Biology of Frankia strain, actinomycetes symbionts of actinorrhizal plants. Microbiol Rev 57:293–319
Bérdy J (2005) Bioactive microbial metabolites. J Antibiot 58:1–26
Bulgari D, Casati P, Brusetti L, Quaglino F, Brasca M, Daffonchio D, Bianco PA (2009) Endophytic bacterial diversity in Grapevine (Vitis vinifera L.) leaves described by 16S rRNA gene sequence analysis and length heterogeneity-PCR. J Microbiol 47:393–401
Cao LX, Qiu ZQ, You JL, Tan HM, Zhou SN (2004) Isolation and characterization of endophytic Streptomyces strains from surfacesterilized tomato (Lycopersicon esculentum) roots. Lett Appl Microbiol 39:425–430
Cao LX, Qiu ZQ, You JL, Tan HM, Zhou S (2005) Isolation and characterization of endophytic streptomycete antagonists of fusarium wilt pathogen from surface-sterilized banana roots. FEMS Microbiol Lett 247:147–152
Caruso M, Colombo AL, Crespi-Perellino N, Fedeli L, Malyszko J, Pavesi A, Quaroni S, Saracchi M, Ventrella G (2000) Studies on a strain of Kitasatospora sp. paclitaxel producer. Ann Microbiol 50:89–102
Castillo UF, Strobel GA, Ford EJ, Hess WM, Porter H, Jensen JB, Albert H, Robison R, Condron MAM, Teplow DB, Stevens D, Yaver D (2002) Munumbicins, wide-spectrum antibiotics produced by Streptomyces NRRL 30562, endophytic on Kennedia nigriscans. Microbiology 148:2675–2685
Castillo U, Harper JK, Strobel GA, Sears J, Alesi K, Ford E, Lin J, Hunter M, Maranta M, Ge H, Yaver D, Jensen JB, Porter H, Robison R, Miller D, Hess WM, Condron M, Teplow D (2003) Kakadumycins, novel antibiotics from Streptomyces sp. NRRL 30566, an endophyte of Grevillea pteridifolia. FEMS Microbiol Lett 234:183–190
Castillo UF, Strobel GA, Mullenberg K, Condron MM, Teplow DB, Folgiano V, Gallo M, Ferracane R, Mannina L, Viel S, Codde M, Robison R, Porter H, Jensen J (2006) Munumbicins E-4 and E-5: novel broad-spectrumantibiotics from Streptomyces NRRL3052. FEMS Microbiol Lett 255:296–300
Chen HH, Qin S, Lee JC, Kim CJ, Xu LH, Jiang CL, Li WJ (2009a) Streptomyces mayteni sp. nov., a novel endophytic actinomycete isolated from Chinese medicinal plant. Antonie Leeuwenhoek 95:47–53
Chen HH, Qin S, Li J, Zhang YQ, Xu LH, Jiang CL, Kim CJ, Li WJ (2009b) Pseudonocardia endophytica sp. nov., isolated from a pharmaceutical plant Lobelia clavata. Int J Syst Evol Microbiol 59:559–593
Chen HH, Zhao GZ, Park DJ, Zhang YQ, Lee JC, Kim CJ, Xu LH, Li WJ (2009c) Micrococcus endophyticus sp. nov., isolated from surface-sterilized Aquilaria sinensis roots. Int J Syst Evol Microbiol 59:1070–1075
Compant S, Duffy B, Nowak J, Clément C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959
Compant S, Clément C, Sessitsch A (2010) Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42:669–678
Conn VM, Franco CMM (2004) Analysis of the endophytec actinobacterial population in the roots of wheat (Triticum aestivum L.) by terminal restriction fragment length polymorphism and sequencing of 16S rRNA clones. Appl Environ Microbiol 70:1787–1794
Conn VM, Walker AR, Franco CM (2008) Endophytic actinobacteria induce defense pathways in Arabidopsis thaliana. Mol Plant Microbe Interact 21:208–218
Coombs JT, Franco CMM (2003) Isolation and identification of actinobacteria isolated from surface-sterilized wheat roots. Appl Environ Microbiol 69:5603–5608
Coombs JT, Michelsen PP, Franco CMM (2004) Evaluation of endophytic actinobacteria as antagonists of Gaeumannomyces graminis var. tritici in wheat. Biol Control 29:359–366
Davis KE, Joseph SJ, Janssen PH (2005) Effects of growth medium, inoculum size, and incubation time on culturability and isolation of soil bacteria. Appl Environ Microbiol 71:826–834
Demain AL, Sanchez S (2009) Microbial drug discovery: 80 years of progress. J Antibiot 62:5–16
Doumbou CL, Akimov V, Beaulieu C (1998) Selection and characterization of microorganisms utilizing Thaxtomin A, a phytotoxin produced by Streptomyces scabies. Appl Environ Microbiol 64:4313–4316
Duangmal K, Thamchaipenet A, Matsumoto A, Takahashi Y (2009) Pseudonocardia acaciae sp. nov., isolated from roots of Acacia auriculiformis A. Cunn. ex Benth. Int J Syst Evol Microbiol 59:1487–1491
El-Gendy MMA, EL-Bondkly AMA (2010) Production and genetic improvement of a novel antimycotic agent, saadamycin, against dermatophytes and other clinical fungi from endophytic Streptomyces sp. Hedaya48. J Ind Microbiol Biotechnol 37(8):831–841
El-Tarabily KA (2003) An endophytic chitinase-producing isolate of Actinoplanes missouriensis, with potential for biological control of root rot of lupine caused by Plectosporium tabacinum. Aust J Bot 51:257–266
El-Tarabily KA, Sivasithamparam K (2006) Non-streptomycete actinomycetes as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters. Soil Biol Biochem 38:1505–1520
El-Tarabily KA, Nassar AH, GEStJ H, Sivasithamparam K (2009) Plant growth promotion and biological control of Pythium aphanidermatum, a pathogen of cucumber, by endophytic actinomycetes. J Appl Microbiol 106:13–26
Ezra D, Castillo UF, Strobel GA, Hess WM, Porter H, Jensen JB, Condron MAM, Teplow DB, Sears J, Maranta M, Hunter M, Weber B, Yaver D (2004) Coronamycins, peptide antibiotics produced by a verticillate Streptomyces sp. (MSU-2110) endophytic on Monstera sp. Microbiology 150:785–793
Fialho de Oliveira M, Germano da Silva M, Van Der Sand ST (2010) Anti-phytopathogen potential of endophytic actinobacteria isolated from tomato plants (Lycopersicon esculentum) in southern Brazil, and characterization of Streptomyces sp. R18(6), a potential biocontrol agent. Res Microbiol 161:565–572
Garcia LC, Martinez-Molina E, Trujillo ME (2010) Micromonospora pisi sp. nov., isolated from root nodules of Pisum sativum. Int J Syst Evol Microbiol 60:331–337
Glick BR, Todorovic B, Czarny J, Cheng Z, Duan J, McConkey B (2007) Promotion of plant growth by bacterial ACC deaminase. Crit Rev Plant Sci 26:227–242
Goodfellow M, Fiedler HP (2010) A guide to successful bioprospecting: informed by actinobacterial systematics. Antonie Leeuwenhoek 98(2):119–142
Gu Q, Luo HL, Zheng W, Liu ZH, Huang Y (2006) Pseudonocardia oroxyli sp. nov., isolated from Oroxylum indicum root. Int J Syst Evol Microbiol 56:2193–2197
Gu Q, Zheng W, Huang Y (2007) Glycomyces sambucus sp. nov., an endophytic actinomycete isolated from the stem of Sambucus adnata Wall. Int J Syst Evol Microbiol 57:1995–1998
Guan S, Suttler I, Lin W, Guo D, Grabley S (2005) p-Aminoacetophenonic acids produced by a mangrove endophyte: Streptomyces griseus subsp. J Nat Prod 68:1198–1200
Gunatilaka AAL (2006) Natural products from plant-associated microorganisms: distribution, structural diversity, bioactivity, and implications of their occurrence. J Nat Prod 69:509–526
Guo B, Wang Y, Sun X, Tang K (2008) Bioactive natural products from endophytes: a review. Appl Biochem Microbiol 44:153–158
Hallmann J, Kloepper JW, Rodríguez-Kábana R (1997a) Application of the scholander pressure bomb to studies on endophytic bacteria of plants. Can J Microbiol 43:411–416
Hallmann J, Quadt-Hallmann A, Mahaffee WF, Kloepper JW (1997b) Bacterial endophytes in agricultural crops. Can J Microbiol 43:895–914
Hallmann J, Berg G, Schulz B (2006) Isolation procedures for endophytic microorganisms. In: Schulz BJE, Boyle CJC, Sieber TN (eds) Microbial root endophytes. Springer, New York, pp 299–314
Hasegawa S, Meguro A, Shimizu M, Nishimura T, Kunoh H (2006) Endophytic actinomycetes and their interactions with host plants. Actinomycetologica 20:72–81
Hayakawa M (1990) Selective isolation methods and distribution of soil actinomycetes. Actinomycetologica 4:103–112
Higashide E, Asai M, OotsuK TS, Kozay Y, Hasegawa T, Kishi T, Sugino Y, Yoneda M (1977) Ansamitocins, a group of novel maytansinoid antibiotics with antitumour properties from Nocardia. Nature 270:721–722
Hirsch AM, Valdés M (2010) Micromonospora: an important microbe for biomedicine and potentially for biocontrol and biofuels. Soil Biol Biochem 42:536–542
Hornung A, Bertazzo M, Dziarnowski A, Schneider K, Welzel K, Wohlert SE, Holzenkämpfer M, Nicholson GJ, Bechthold A, Süssmuth RD, Vente A, Pelzer S (2007) A genomic screening approach to the structure-guided identification of drug candidates from natural sources. Chembiochem 8:757–766
Igarashi Y (2004) Screening of novel bioactive compounds from plant-associated actinomycetes. Actinomycetologica 18:63–66
Igarashi Y, Iida T, Yoshida R, Furumai T (2002) Pteridic acids A and B, novel plant growth promoters with auxin-like activity from Streptomyces hygroscopicus TP-A0451. J Antibiot 55:764–767
Igarashi Y, Miura S, Fujita T, Furumai T (2006) Pterocidin, a cytotoxic compound from the endophytic Streptomyces hygroscopicus. J Antibiot 59:193–195
Igarashi Y, Trujillo ME, Martínez-Molina E, Miyanaga S, Obata T, Sakurai H, Saiki I, Fujita T, Furumai T (2007) Antitumor anthraquinones from an endophytic actinomycete Micromonospora lupini sp. nov. Bioorg Med Chem Lett 17:3702–3705
Ikeda S, Kaneko T, Okubo T, Rallos LEE, Eda S, Mitsui H, Sato S, Nakamura Y, Tabata S, Minamisawa K (2009) Development of a bacterial cell enrichment method and its application to the community analysis in soybean stems. Microb Ecol 58:703–714
Ikeda S, Okubo T, Kaneko T, Inaba S, Maekawa T, Eda S, Sato S, Tabata S, Mitsui H, Minamisawa K (2010) Community shifts of soybean stem-associated bacteria responding to different nodulation phenotypes and N levels. ISME J 4:315–326
Inahashi Y, Matsumoto A, Danbara H, Ōmura S, Takahashi Y (2009) Phytohabitans suffuscus gen. nov., sp. nov., a novel actinomycete of the family Micromonosporaceae isolated from a plant root. Int J Syst Evol Microbiol. doi:10.1099/ijs.0.016477-0
Indananda C, Matsumoto A, Inahashi Y, Takahashi Y, Duangmal K, Thamchaipenet A (2010a) Actinophytocola gen. nov., a new genus of the family Pseudonocardiaceae and description of a new species, Actinophytocola oryzae sp. nov., isolated from root of Thai glutinous rice plant. Int J Syst Evol Microbiol 60:1141–1146
Indananda C, Thamchaipenet A, Matsumoto A, Duangmal K, Takahashi Y (2010b) Actinoallomurus oryzae sp. nov., an endophytic actinomycete isolated from root of Thai jasmine rice plant. Int J Syst Evol Microbiol. doi:10.1099/ijs.0.022509-0
Janso JE, Carter GT (2010) Biosynthetic potential of phylogenetically unique endophytic actinomycetes from tropical plants. Appl Environ Microbiol 76:4377–4386
Jiao JY, Wang HX, Zeng Y, Shen YM (2006) Enrichment for microbes living in association with plant tissues. J Appl Microbiol 100:830–837
Kaewkla O, Franco CMM (2010a) Nocardia callitridis sp. nov., an endophytic actinobacterium isolated from a surface-sterilized root of an Australian native pine tree. Int J Syst Evol Microbiol 60:1532–1536
Kaewkla O, Franco CMM (2010b) Pseudonocardia adelaidensis sp. nov., an endophytic actinobacterium isolated from the surface-sterilized stem of a Grey Box tree. Int J Syst Evol Microbiol. doi:10.1099/ijs.0.019208-0
Kaewkla O, Franco CMM (2010c) Pseudonocardia eucalypti sp. nov., an endophytic actinobacterium with a unique knobby spore surface, isolated from the surface-sterilized root of a native Australian eucalyptus tree. Int J Syst Evol Microbiol. doi:10.1099/ijs.0.022327-0
Kannan V, Sureendar R (2008) Synergistic effect of beneficial rhizosphere microflora in biocontrol and plant growth promotion. J Basic Microbiol 49:158–164
Kim N, Shin JC, Kim W, Hwang BY, Kim BS, Hong YS, Lee D (2006) Cytotoxic 6-alkylsalicylic acids from the endophytic Streptomyces laceyi. J Antibiot 59:797–800
Krause A, Ramakumar A, Bartels D, Battistoni F, Bekel T, Boch J, Böhm M, Friedrich F, Hurek T, Krause L, Linke B, McHardy AC, Sarkar A, Schneiker S, Syed AA, Thauer R, Vorhölter FJ, Weidner S, Pühler A, Reinhold-Hurek B, Kaiser O, Goesmann A (2006) Complete genome of the mutualistic N2-fixing grass endophyte Azoarcus sp. strain BH72. Nat Biotechnol 24:1384–1390
Krechel A, Faupel A, Hallmann J, Ulrich A, Berg G (2002) Potato associated bacteria and their antagonistic potential towards plant-pathogenic fungi and the plant-parasitic nematode Meloidogyne incognita (Kofoid & White) Chitwood. Can J Microbiol 48:772–786
Kupchan SM, Komoda Y, Court WA, Thomas GJ, Smith RM, Karim A, Gilmore CJ, Haltiwanger RC, Bryan RF (1972) Maytansine, a novel antileukaemic ansa macrolide from Maytenus ovatus. J Am Chem Soc 94:1355–1356
Küster E, Williams ST (1964) Media for the isolation of streptomycetes: starch casein medium. Nature 202:928–929
Lauber CL, Zhou N, Gordon JI, Knight R, Fierer N (2010) Effect of storage conditions on the assessment of bacterial community structure in soil and human-associated samples. FEMS Microbiol Lett 307(1):80–86
Lee SO, Choi GJ, Choi YH, Jang KS, Park DJ, Kim CJ, Kim JC (2008) Isolation and characterization of endophytic actinomycetes from Chinese cabbage roots as antagonists to Plasmodiophora brassicae. J Microbiol Biotechnol 18:1741–1746
Lewis K, Epstein S, D’Onofrio A, Ling LL (2010) Uncultured microorganisms as a source of secondary metabolites. J Antibiot 63:468–476
Li J, Zhao GZ, Chen HH, Qin S, Xu LH, Jiang CL, Li WJ (2008a) Rhodococcus cercidiphylli sp. nov., a new endophytic actinobacterium isolated from leaf of Cercidiphyllum japonicum. Syst Appl Microbiol 31:1108–1113
Li J, Zhao GZ, Zhang YQ, Klenk HP, Pukall R, Qin S, Xu LH, Li WJ (2008b) Dietzia schimae sp. nov. and Dietzia cercidiphylli sp. nov., from surface-sterilized plant tissues. Int J Syst Evol Microbiol 58:2549–2554
Li J, Zhao GZ, Huang HY, Qin S, Zhu WY, Li WJ (2009a) Kineosporia mesophila sp. nov., isolated from the surface-sterilized stem of Tripterygium wilfordii. Int J Syst Evol Microbiol 59:3150–3154
Li J, Zhao GZ, Qin S, Huang HY, Zhu WY, Xu LH, Li WJ (2009b) Saccharopolyspora tripterygii sp. nov., an endophytic actinomycete isolated from the stem of Tripterygium hypoglaucum. Int J Syst Evol Microbiol 59:3040–3044
Li J, Zhao GZ, Qin S, Zhu WY, Xu LH, Li WJ (2009c) Herbidospora osyridis sp. nov., isolated from tissue of Osyris wightiana Wall. Int J Syst Evol Microbiol 59:3123–3127
Li J, Zhao GZ, Qin S, Zhu WY, Xu LH, Li WJ (2009d) Streptomyces sedi sp. nov., isolated from surface-sterilized roots of Sedum sp. Int J Syst Evol Microbiol 59:1492–1496
Li J, Lu CH, Shen YM (2010a) Macrolides of the bafilomycin family produced by Streptomyces sp. CS. J Antibiot. doi:10.1038/ja.2010.95
Li J, Zhao GZ, Huang HY, Zhu WY, Lee JC, Xu LH, Kim CJ, Li WJ (2010b) Nonomuraea endophytica sp. nov., an endophytic actinomycete isolated from Artemisia annua L. Int J Syst Evol Microbiol. doi:10.1099/ijs.0.022558-0
Lin ZJ, Lu XM, Zhu TJ, Fang YC, Gu QQ, Zhu WM (2008) GPR12 Selections of the metabolites from an endophytic Streptomyces sp. asociated with Cistanches deserticola. Arch Pharm Res 31:1108–1114
Liu N, Wang HB, Liu M, Gu Q, Zheng W, Huang Y (2009) Streptomyces alni sp. nov., a daidzein-producing endophyte isolated from a root of Alnus nepalensis D. Don. Int J Syst Evol Microbiol 59:254–258
Lu CH, Shen YM (2003) A new macrolide antibiotics with antitumor activity produced by Streptomyces sp. CS, a commensal microbe of Maytenus hookeri. J Antibiot 56:415–418
Lu CH, Shen YM (2004) Two new macrolides produced by Streptomyces sp. CS. J Antibiot 57:597–600
Lu CH, Shen YM (2007) A novel ansamycin, naphthomycin K from Streptomyces sp. J Antibiot 60:649–653
Manter DK, Delgado JA, Holm DG, Stong RA (2010) Pyrosequencing reveals a highly diverse and cultivar-specific bacterial endophyte community in potato roots. Microb Ecol 60:157–166
Mardis ER (2008) Next-generation DNA sequencing methods. Annu Rev Genomics Hum Genet 9:387–402
Merzaeva OV, Shirokikh IG (2010) The production of auxins by the endophytic bacteria of winter rye. Appl Biochem Microbiol 46:44–50
Miller SR, Strong AL, Jones KL, Ungerer MC (2009) Bar-coded pyrosequencing reveals shared bacterial community properties along the temperature gradients of two alkaline hot springs in Yellowstone National Park. Appl Environ Microbiol 75:4565–4572
Moore BS, Kalaitzis JA, Xiang L (2005) Exploiting marine actinomycete biosynthetic pathways for drug discovery. Antonie Leeuwenhoek 87:49–57
Nejad P, Johnson PA (2000) Endophytic bacteria induce growth promotion and wilt disease suppression in oilseed rape and tomato. Biol Control 18:208–215
Newman DJ, Cragg GM (2007) Natural products as sources of new drugs over the last 25 years. J Nat Prod 70:461–477
Nimnoi P, Pongsilp N, Lumyong S (2010) Endophytic actinomycetes isolated from Aquilaria crassna Pierre ex Lec and screening of plant growth promoters production. World J Microbiol Biotechnol 26:193–203
Okazaki T (2003) Studies on actinomycetes isolated from plant leaves. In: Kurtböke DI (ed) Selective isolation of rare actinomycetes. Queensland Complete Printing Service, Australia, pp 102–121
Powel RG, Weisleder D, Smith CR, Kozlowski J, Rohwedder WK (1982) Treflorine, trenudine, and Nmethyltrenudone: novel maytansinoids tumour inhibitors containing two fused macrocyclic rings. J Am Chem Soc 104:4929–4934
Pullen C, Schmitz P, Meurer K, Bamberg DD, Lohmann S, Franc SDC, Groth I, Schlegel B, Möllmann U, Gollmick F, Gräfe U, Leistner E (2002) New and bioactive compounds from Streptomyces strains residing in the wood of Celastraceae. Planta 216:162–167
Qin S, Li J, Zhao GZ, Chen HH, Xu LH, Li WJ (2008a) Scharopolyspora endophytica sp. nov., an endophytic actinomycete isolated from the root of Maytenus austroyunnanensis. Syst Appl Microbiol 31:352–357
Qin S, Wang HB, Chen HH, Zhang YQ, Jiang CL, Xu LH, Li WJ (2008b) Glycomyces endophyticus sp. nov., an endophytic actinomycete isolated from the root of Carex baccans Nees. Int J Syst Evol Microbiol 58:2525–2528
Qin S, Chen HH, Klenk HP, Zhao GZ, Li J, Xu LH, Li WJ (2009a) Glycomyces scopariae sp. nov. and Glycomyces mayteni sp. nov., isolated from two medicinal plants in China. Int J Syst Evol Microbiol 59:1023–1027
Qin S, Li J, Chen HH, Zhao GZ, Zhu WY, Jiang CL, Xu LH, Li WJ (2009b) Isolation, diversity, and antimicrobial activity of rare actinobacteria from medicinal plants of tropical rain forests in Xishuangbanna, China. Appl Environ Microbiol 75:6176–6186
Qin S, Li J, Zhang YQ, Zhu WY, Zhao GZ, Xu LH, Li WJ (2009c) Plantactinospora mayteni gen. nov., sp. nov., a member of the family Micromonosporaceae. Int J Syst Evol Microbiol 59:2527–2533
Qin S, Zhao GZ, Klenk HP, Li J, Xu LH, Li WJ (2009d) Nonomuraea antimicrobica sp. nov., an endophytic actinomycete isolated from leaves of Maytenus austroyunnanensis. Int J Syst Evol Microbiol 59:2453–2457
Qin S, Zhao GZ, Li J, Zhu WY, Xu LH, Li WJ (2009e) Actinomadura flavalba sp. nov., an endophytic actinomycete isolated from leaves of Maytenus austroyunnanensis. Int J Syst Evol Microbiol 59:2453–2457
Qin S, Zhao GZ, Li J, Zhu WY, Xu LH, Li WJ (2009f) Jiangella alba sp. nov., an endophytic actinomycete isolated from the stem of Maytenus austroyunnanensis. Int J Syst Evol Microbiol 59:2162–2165
Qin S, Zhu WY, Jiang JH, Klenk HP, Li J, Zhao GZ, Xu LH, Li WJ (2009g) Pseudonocardia tropica sp. nov., a novel endophytic actinomycete isolated from the stem of Maytenus austroyunnanensis. Int J Syst Evol Microbiol. doi:10.1099/ijs.0.020099-0
Qin S, Chen HH, Lee JC, Kim CJ, Xu LH, Li WJ (2010a) Sccharopolyspora gloriosa sp. nov., a novel endophytic actinomycete isolated from the stem of Gloriosa superba L. Int J Syst Evol Microbiol 60:1147–1151
Qin S, Xing K, Fei SM, Lin Q, Chen XM, Cao CL, Sun Y, Wang Y, Li WJ, Jiang JH (2010b) Pseudonocardia sichuanensis sp. nov., a novel endophytic actinomycete isolated from the root of Jatropha curcas L. Antonie Leeuwenhoek. doi:10.1007/s10482-010-9504-7
Qiu FB, Huang Y, Sun L, Zhang XX, Liu ZH, Song W (2007) Leifsonia ginsengi sp. nov., isolated from ginseng root. Int J Syst Evol Microbiol 57:405–408
Robinson CJ, Bohannan BJ, Young VB (2010) From structure to function: the ecology of host-associated microbial communities. Microbiol Mol Biol Rev 74(3):453–476
Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN (2008) Bacterial endophytes: recent development and applications. FEMS Micribiol Lett 278:1–9
Sessitsch A, Reiter B, Berg G (2004) Endophytic bacterial communities of field-grown potato plants and their plant-growth promoting and antagonistic abilities. Can J Microbiol 50:239–249
Shendure J, Ji H (2008) Next-generation DNA sequencing. Nat Biotechnol 26:1135–1145
Shimizu M, Suzuki T, Mogami O, Kunoh H (2005) Disease resistance of plants induced by endophytic actinomycetes. In: Tsuyumu S, Leach JE, Shiraishi T, Wolpert T (eds) Genomic and genetic analysis of plant parasitism and defense. APS, St. Paul, pp 292–293
Shimizu M, Yazawa S, Ushijima Y (2009) A promising strain of endophytic Streptomyces sp. for biological control of cucumber anthracnose. J Gen Plant Pathol 75:27–36
Shirling EB, Gottlieb D (1966) Methods for characterization of Streptomyces species. Int J Syst Bacteriol 16:313–340
Snipes CE, Duebelbeis DO, Olson M, Hahn DR, Dent WH, Gilbert JR, Werk TL, Davis GE, Lee-Lu R, Graupner PR (2007) The ansacarbamitocins: polar ansamitocin derivatives. J Nat Prod 70:1578–1581
Song GC, Yasir M, Bibi F, Chung EJ, Jeon CO, Chung YR (2010) Nocardioides caricicola sp. nov., an endophytic bacterium isolated from a halophyte, Carex scabrifolia Steud. Int J Syst Evol Microbiol. doi:10.1099/ijs.0.019919-0
Staniek A, Woerdenbag HJ, Kayser O (2008) Endophytes: exploiting biodiversity for the improvement of natural product-based drug discovery. J Plant Interact 3:75–93
Stone JK, Bacon CW, White JF (2000) An overview of endophytic microbes: endophytism defined. In: Bacon CW, White JF (eds) Microbial endophytes. Marcel Dekker Inc., New York, pp 3–29
Strobel GA, Daisy B (2003) Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev 67:491–502
Strobel G, Daisy B, Castillo U, Harper J (2004) Natural products from endophytic microorganisms. J Nat Prod 67:257–268
Sturz AV (1995) The role of endophytic bacteria during seed piece decay and potato tuberization. Plant Soil 175:257–263
Sun Y, Cheng Z, Glick BR (2009) The presence of a 1-aminocyclopropane-1-carboxylate (ACC) deaminase deletion mutation alters the physiology of the endophytic plant growth promoting bacterium Burkholderia phytofirmans PsJN. FEMS Microbiol Lett 296:131–136
Surette MA, Sturz AV, Lada RR, Nowak J (2003) Bacterial endophytes in processing carrots (Daucus carota L. var. sativus): their localization, population density, biodiversity and their effects on plant growth. Plant Soil 253:381–390
Suryanarayanan TS, Thirunavukkarasu N, Govindarajulu MB, Sasse F, Jansen R, Murali TS (2009) Fungal endophytes and bioprospecting. Fungal Biol Rev 23:9–19
Suwanborirux K, Chang CJ, Spjut RW, Cassady JM (1990) Ansamitocin P-3, a maytansinoid from Claopodium crispifolium and Anomodon attenuatus or associated actinomycetes. Experientia 46:117–120
Taechowisan T, Peberdy JF, Lumyong S (2003) Isolation of endophytic actinomycetes from selected plants and their antifungal activity. World J Microbiol Biotechnol 19:381–385
Taechowisan T, Lu C, Shen Y, Lumyong S (2005) Secondary metabolites from endophytic Streptomyces aureofaciens CMUAc130 and their antifungal activity. Microbiology 151:1691–1695
Taechowisan T, Wanbanjob A, Tuntiwachwuttikul P, Taylor WC (2006) Identification of Streptomyces sp. Tc022, an endophyte in Alpinia galanga, and the isolation of actinomycin D. Ann Microbiol 56(2):113–117
Taechowisan T, Lu CH, Shen YM, Lumyong S (2007a) 4-arylcoumarin inhibits immediate-type allergy. Food Agric Immunol 18:203–211
Taechowisan T, Lu CH, Shen YM, Lumyong S (2007b) Antitumor activity of 4-arylcoumarins from endophytic Streptomyces aureofaciens CMUAc130. J Cancer Res Trer 3:86–91
Taechowisana T, Wanbanjob A, Tuntiwachwuttikul P, Liu JK (2009) Anti-inflammatory activity of lansais from endophytic Streptomyces sp. SUC1 in LPS-induced RAW 264.7 cells. Food Agric Immunol 20:67–77
Tan HM, Cao LX, He ZF, Su GJ, Lin B, Zhou SN (2006) Isolation of endophytic actinomycetes from different cultivars of tomato and their activities against Ralstonia solanacearum in vitro. World J Microbiol Biotechnol 22:1275–1280
Thamchaipenet A, Indananda C, Bunyoo C, Duangmal K, Matsumoto A, Takahashi Y (2010) Actinoallomurus acaciae sp. nov., a novel endophytic actinomycete isolated from Acacia auriculiformis A. Cunn. ex Benth. in Thailand. Int J Syst Evol Microbiol 60:554–559
Tian XL, Cao LX, Tan HM, Han WQ, Chen M, Liu YH, Zhou SN (2007) Diversity of cultivated and uncultivated actinobacterial endophytes in the stems and roots of rice. Microb Ecol 53:700–707
Trujillo ME, Kroppenstedt RM, Schumann P, Carro L, Martínez-Molina E (2006) Micromonospora coriariae sp. nov., isolated from root nodules of Coriaria myrtifolia. Int J Syst Evol Microbiol 56:2381–2385
Trujillo ME, Kroppenstedt RM, Fernández-Molinero C, Schumann P, Martínez-Molina E (2007) Micromonospora lupini sp. nov. and Micromonospora saelicesensis sp. nov., isolated from root nodules of Lupinus angustifolius. Int J Syst Evol Microbiol 57:2799–2804
Tuntiwachwuttikul P, Taechowisan T, Wanbanjob A, Thadaniti S, Taylor WC (2008) Lansai A–D, secondary metabolites from Streptomyces sp. SUC1. Tetrahedron 64:7583–7586
Van Overbeek L, Van Elsas JD (2008) Effects of plant genotype and growth stage on the structure of bacterial communities associated with potato (Solanumtuberosum L.). FEMS Microbiol Ecol 64:283–296
Velazquez E, Rojas M, Lorite MJ, Rivas R, Zurdo-Pineiro JL, Heydrich M, Bedmar EJ (2008) Genetic diversity of endophytic bacteria which could be find in the apoplastic sap of medullary parenchym of the stem of healthy sugarcane plants. J Basic Microbiol 48:118–124
Verma VC, Gond SK, Kumar A, Mishra A, Kharwar RN, Gange AC (2009a) Endophytic actinomycetes from Azadirachta indica A. Juss: isolation, diversity, and anti-microbial activity. Microb Ecol 57:749–756
Verma VC, Kharwar RN, Strobel GA (2009b) Chemical and functional diversity of natural products from plant associated endophytic fungi. Nat Prod Commun 11:1511–1532
Vickers JC, Williams ST, Ross GW (1984) A taxonomic approach to selective isolation of streptomycetes from soil. In: Ortiz-Ortiz L, Bojalil LF, Yakoleff V (eds) Biological, biochemical and biomedical aspects of actinomycetes. Academic, Orlando, pp 553–561
Wang HX, Geng ZL, Zeng Y, Shen YM (2008) Enrichment plant microbiota for a metagenomic library construction. Environ Microbiol 10:2684–2691
Wu X, Monchy S, Taghavi S, Zhu W, Ramos J, van der Lelie D (2010) Comparative genomics and functional analysis of niche-specific adaptation in Pseudomonas putida. FEMS Microbiol Rev. doi:10.1111/j.1574-6976.2010.00249.x
Xie QY, Wang C, Wang R, Qu Z, Lin HP, Goodfellow M, Hong K (2010) Jishengella endophytica gen. nov., sp. nov., a new member of the family Micromonosporaceae. Int J Syst Evol Microbiol. doi:10.1099/ijs.0.025288-0
Xing K, Qin S, Fei SM, Lin Q, Bian GK, Miao Q, Wang Y, Cao CL, Tang SK, Jiang JH, Li WJ (2010) Nocardia endophytica sp. nov., a novel endophytic actinomycete isolated from oil-seed plant Jatropha curcas L. Int J Syst Evol Microbiol. doi:10.1099/ijs.0.027391-0
Yan LL, Han NN, Zhang YQ, Yu LY, Chen J, Wei YZ, Li QP, Tao L, Zheng GH, Yang SE, Jiang CX, Zhang XD, Huang Q, Habdin X, Hu QB, Li Z, Liu SW, Zhang ZZ, He QY, Si SY, Sun CH (2010) Antimycin A18 produced by an endophytic Streptomyces albidoflavus isolated from a mangrove plant. J Antibiot 63:259–261
Yuan HM, Zhang XP, Zhao K, Zhong K, Gu YF, Lindstrom K (2008) Genetic characterisation of endophytic actinobacteria isolated from the medicinal plants in Sichuan. Ann Microbiol 58(4):597–604
Zhang HW, Song YC, Tan RX (2006) Biology and chemistry of endophytes. Nat Prod Rep 23:753–771
Zhao PJ, Fan LM, Li GH, Zhu N, Shen YM (2005) Antibacterial and antitumor macrolides from Streptomyces sp. ls9131. Arch Pharm Res 28:1228–1232
Zhao GZ, Li J, Qin S, Zhang YQ, Zhu WY, Jiang CL, Xu LH, Li WJ (2009) Micrococcus yunnanensis sp. nov., a novel actinobacterium isolated from surface-sterilized Polyspora axillaris root. Int J Syst Evol Microbiol 59:2383–2387
Zhao GZ, Li J, Huang HY, Zhu WY, Zhao LX, Tang SK, Xu LH, Li WJ (2010a) Pseudonocardia artemisiae sp. nov., a novel actinobacterium isolated from surface-sterilized Artemisia annua L. Int J Syst Evol Microbiol. doi:10.1099/ijs.0.021931-0
Zhao GZ, Li J, Qin S, Huang HY, Zhu WY, Xu LH, Li WJ (2010b) Streptomyces artemisiae sp. nov., a novel actinomycete isolated from surface-sterilized Artemisia annua L. tissue. Int J Syst Evol Microbiol 60:27–32
Zhao K, Penttinen P, Guan TW, Xiao J, Chen Q, Xu J, Lindström K, Zhang LL, Zhang XP, Strobel GA (2010c) The diversity and anti-microbial activity of endophytic actinomycetes isolated from medicinal plants in Panxi plateau, China. Curr Microbiol. doi:10.1007/s00284-010-9685-3
Zhu N, Zhao P, Shen Y (2009) Selective isolation and ansamycin-targeted screenings of commensal actinomycetes from the “maytansinoids-producing” arboreal Trewia nudiflora. Curr Microbiol 58:87–94
Zin NM, Sarmin NIM, Ghadin N, Basri DF, Sidik NM, Hess WM, Strobel GA (2007) Bioactive endophytic streptomycetes from the Malay Peninsula. FEMS Microbiol Lett 274:83–88
Acknowledgements
This work was partially supported by the National Basic Research Program of China (No. 2010CB833800), National Natural Science Foundation of China (Project no. 30872028, 31000005), the Major Fundamental Research Program of Natural Science Foundation of the Jiangsu Higher Education Institutions of China (08KJA350001), the Program of the Demonstration and Study of Standardization Seeding Technology of Jatropha (2007BAD50B0204) and Grants from Natural Science Foundation by Xuzhou Normal University (09XLR12, 09XLR19).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Qin, S., Xing, K., Jiang, JH. et al. Biodiversity, bioactive natural products and biotechnological potential of plant-associated endophytic actinobacteria. Appl Microbiol Biotechnol 89, 457–473 (2011). https://doi.org/10.1007/s00253-010-2923-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2923-6