Abstract
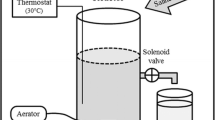
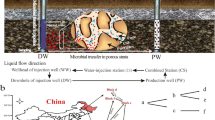
Injection of up-flow packed-bed bioreactors with excess volatile fatty acids and limiting concentrations of nitrate and sulfate gave complete reduction of nitrate from 0 to 5.5 cm and complete or near-complete reduction of sulfate from 3.2 to 11.5 cm along the bioreactor flow path. Most of the biomass (85%) and most of the genes for nitrate reduction (narG, 96%; napA, 99%) and for sulfate reduction (dsrB, 91%) were present near the inlet (0–5.5 cm) of the 37-cm-long bioreactor. Microbial community analysis by a combination of denaturing gradient gel electrophoresis and pyrosequencing of 16S rRNA amplicons indicated that nitrate-reducing Arcobacter and Pseudomonas species were located from 0 to 3.2 cm and throughout, respectively. Desulfobulbus species were the main sulfate reducers present and acetotrophic methanogens of the genus Methanosaeta predominated at 20–37 cm. Overall, the results indicated a succession of microbial communities along the bioreactor flow path. In the absence of nitrate, the sulfate reduction zone moved nearer to the bioreactor inlet. The sulfide concentration in the bioreactor effluent was temporarily lowered after nitrate injection was re-started. Hence, the bioreactor sulfide output could be disrupted by pulsed, not by constant nitrate injection, as demonstrated also previously in a low-temperature oil field.







Similar content being viewed by others
References
Agrawal A, Lal B (2009) Rapid detection and quantification of bisulfite reductase genes in an oil field using real time-PCR. FEMS Microbiol Ecol 69:301–312
Bødtker G, Lysnes K, Torsvik T, Eø B, Sunde E (2009) Microbial analysis of backflowed injection water from a nitrate-treated North Sea oil reservoir. J Ind Microbiol Biotechnol 36:439–450
Bru D, Sarr A, Philippot L (2007) Relative abundance of proteobacterial membrane-bound and periplasmic nitrate reductases in selected environments. Appl Environ Microbiol 73:5971–5974
Cornish Shartau SL, Yurkiw M, Lin S, Grigoryan AA, Lambo A, Park HS, Lomans BP, van der Biezen E, Jetten MSM, Voordouw G (2010) Ammonium concentrations in produced waters from a mesothermic oil field subjected to nitrate injection decrease through formation of denitrifying biomass and anammox activity. Appl Environ Microbiol 76:4977–4987
Gevertz D, Telang AJ, Voordouw G, Jenneman GE (2000) Isolation and characterization of strains CVO and FWKO B, two novel nitrate-reducing, sulfide-oxidizing bacteria isolated from oil field brine. Appl Environ Microbiol 66:2491–2501
Gittel A, Sorensen KB, Skovhus TL, Ingvorsen K, Schramm A (2009) Prokaryotic community structure and activity of sulfate reducers in production water from high-temperature oil reservoirs with and without nitrate treatment. Appl Environ Microbiol 75:7086–7096
Greene EA, Hubert C, Nemati M, Jenneman GE, Voordouw G (2002) Nitrite reductase activity of sulfate-reducing bacteria prevents their inhibition by nitrate-reducing, sulfide-oxidizing bacteria. Environ Microbiol 5:607–617
Grigoryan AA, Cornish SL, Buziak B, Lin S, Cavallaro A, Arensdorf JJ, Voordouw G (2008) Competitive oxidation of volatile fatty acids by sulfate- and nitrate-reducing bacteria from an oil field in Argentina. Appl Environ Microbiol 74:4324–4335
Hamady M, Lozupone C, Knight R (2010) Fast UniFrac: facilitating high-throughput phylogenetic analyses of microbial communities including analysis of pyrosequencing and PhyloChip data. ISME J 4:17–27
Hubert CM, Nemati M, Jenneman GE, Voordouw G (2003) Containment of biogenic sulfide production in continuous up-flow packed-bed bioreactors with nitrate or nitrite. Biotechnol Prog 19:338–345
Huse S, Huber J, Morrison H, Sogin M, Welch D (2007) Accuracy and quality of massively parallel DNA pyrosequencing. Genome Biol 8(7):R143
Huson D, Richter D, Rausch C, Dezulian T, Franz M, Rupp R (2007) Dendroscope: an interactive viewer for large phylogenetic trees. BMC Bioinformatics 8:460
Karkhoff-Schweizer RR, Huber PW, Voordouw G (1995) The dissimilatory sulfide reductases of the mesophilic eubacterium Desulfovibrio vulgaris and of the thermophilic archaeum Archaeoglobus fulgidus are highly conserved. Appl Environ Microbiol 61:290–296
Marmur J (1961) A procedure for the isolation of deoxyribonucleic acid from microorganisms. J Mol Biol 3:208–218
Moreno-Vivian C, Cabello P, Martinez-Luque M, Blasco R, Castillo F (1999) Prokaryotic nitrate reduction: molecular properties and functional distinction among bacterial nitrate reductases. J Bacteriol 181:6573–6584
Myhr S, Lillebø BL, Sunde E, Beeder J, Torsvik T (2002) Inhibition of H2S producing hydrocarbon degrading bacteria in an oil reservoir model column by nitrate injection. Appl Microbiol Biotechnol 58:400–408
Okabe S, Santegoeds CM, De Beer D (2003) Effect of nitrite and nitrate on in situ sulfide production in an activated sludge immobilized agar gel film as determined by the use of microelectrodes. Biotechnol Bioeng 81:570–577
Philippot L, Hojberg O (1999) Dissimilatory nitrate reductases in bacteria. Biochim Biophys Acta 1446:1–23
Pruesse E, Quast C, Knittel K, Fuchs B, Ludwig W, Peplies J, Glöckner FO (2007) SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucl Acids Res 35:7188–7196
Ramos-Padrón E, Bordenave S, Lin S, Bhaskar IM, Dong X, Sensen CW, Fournier J, Voordouw G, Gieg LM (2011) Carbon and sulfur cycling by microbial communities in a gypsum-treated oil sands tailings pond. Environ Sci Technol 45:439–446
Reinsel MA, Sears JT, Stewart PS, McInerney MJ (1996) Control of microbial souring by nitrate, nitrite or glutaraldehyde injection in a sandstone column. J Ind Microbiol 17:128–136
Schloss PD, Westcott SL, Thomas R, Hall RJR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Sunde E, Torsvik T (2005) Microbial control of hydrogen sulfide production in oil reservoirs. In: Olliver B, Mago M (eds) Petroleum microbiology. ASM, Washington
Telang AJ, Ebert S, Foght JM, Westlake DWS, Jenneman GE, Gevertz D, Voordouw G (1997) The effect of nitrate injection on the microbial community in an oil field as monitored by reverse sample genome probing. Appl Environ Microbiol 63:1785–1793
Trüper HG, Schlegel HG (1964) Sulphur metabolism in Thiorhodaceae I. Quantitative measurements in growing cells of Chromatium okenii. Antonie Leeuwenhoek 30:225–238
Vance I, Thrasher DR (2005) Reservoir souring: mechanisms and prevention. In: Olliver B, Mago M (eds) Petroleum microbiology. ASM, Washington
Vandamme P, Falsen E, Rossau R, Hoste B, Segers P, Tytgat R, De Ley J (1991) Revision of Campylobacter, Helicobacter, and Wolinella taxonomy: emendation of generic descriptions and proposal of Arcobacter gen. nov. Int J Syst Bacteriol 41:88–103
Voordouw G, Armstrong SM, Reimer MF, Fouts B, Telang AJ, Shen Y, Gevertz D (1996) Characterization of 16S rRNA genes from oil field microbial communities indicates the presence of a variety of sulfate-reducing, fermentative, and sulfide-oxidizing bacteria. Appl Environ Microbiol 62:1623–1629
Voordouw G, Grigoryan AA, Lambo A, Lin S, Park HS, Jack TR, Coombe D, Clay B, Zhang F, Ertmoed R, Miner K, Arensdorf JJ (2009) Sulfide remediation by pulsed injection of nitrate into a low temperature Canadian heavy oil reservoir. Environ Sci Technol 43:9512–9518
Widdel F, Pfennig N (1982) Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids II. Incomplete oxidation of propionate by Desulfobulbus propionicus gen. nov., sp. nov. Arch Microbiol 131:360–365
Zumft WG (1997) Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev 61:533–616
Acknowledgments
This work was supported by an NSERC Industrial Research Chair Award to GV, which was also supported by Baker Hughes Incorporated, Commercial Microbiology Limited (Intertek), the Computer Modelling Group Limited, ConocoPhillips Company, YPF SA, Aramco Services, Shell Canada Limited, Suncor Energy Developments Inc., and Yara International ASA, as well as by the Alberta Innovates-Energy and Environment Solutions. This work was also supported by funding from Genome Canada, Genome Alberta, the Government of Alberta, and Genome BC. The authors are grateful for technical support provided by Dr. Sasha Grigoryan and for administrative support by Dr. Rhonda Clark. Gary Leveque from the Genome Quebec and McGill University Innovation Centre, Montreal, Quebec, are thanked for pyrosequencing.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Clustering analysis of pyrosequencing data obtained for samples from bioreactor-C using the Fast UniFrac service based on the weighted and normalized UniFrac unit matrix. The scale bar indicates the distance between clusters in UniFrac units (DOC 34 kb)
Table S1
Class level survey of 16S sequences in samples from bioreactor-C. The number of pyrosequencing reads (N) for each sample is indicated. The percentage fraction (%) of these is listed for each class. The list is ranked in order of most- to least-prevalent class for all samples. Classes with a percentage below 0.02% were eliminated. Samples were obtained for the glass wool (GW), polymeric mesh (PM), and above port 1a (a1a) sections as well as for ports 1a, 1, 2, 3, and 4 of bioreactor-C (see Fig. 1) (DOC 426 kb)
Table S2
Genus level survey of 16S sequences in samples from bioreactor-C. The number of reads (N) for each sample is indicated. The fractions (%) of these are listed for each genus. The list is ranked in order of most to least prevalent genus for all samples. Genera with an average fraction of 0.02% or less were eliminated. Samples were obtained for the glass wool (GW) and polymeric mesh (PM) and above port 1a (a1a) sections, as well as for ports 1a, 1, 2, 3, and 4 of bioreactor-C (see Fig. 1) (DOC 632 kb)
Table S3
Biomass and DNA concentrations in fractions obtained from bioreactor-C (DOC 46 kb)
Rights and permissions
About this article
Cite this article
Callbeck, C.M., Dong, X., Chatterjee, I. et al. Microbial community succession in a bioreactor modeling a souring low-temperature oil reservoir subjected to nitrate injection. Appl Microbiol Biotechnol 91, 799–810 (2011). https://doi.org/10.1007/s00253-011-3287-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3287-2